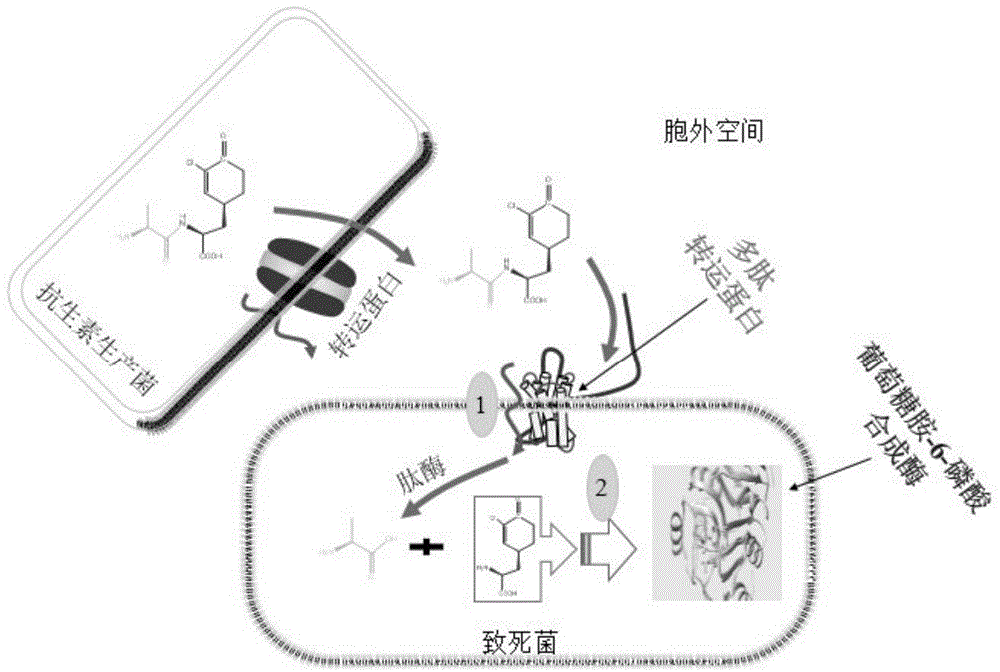
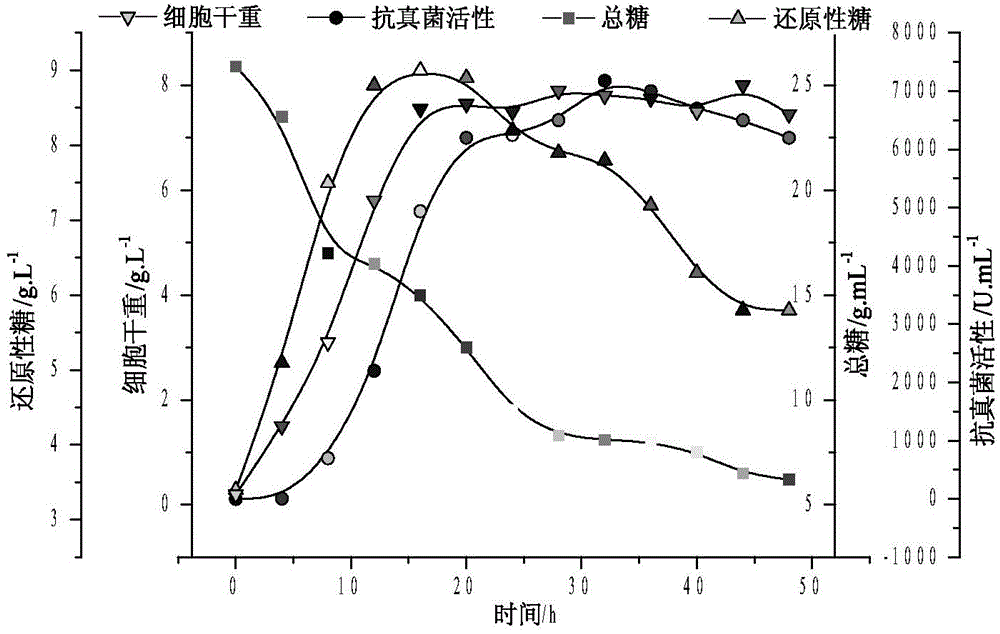
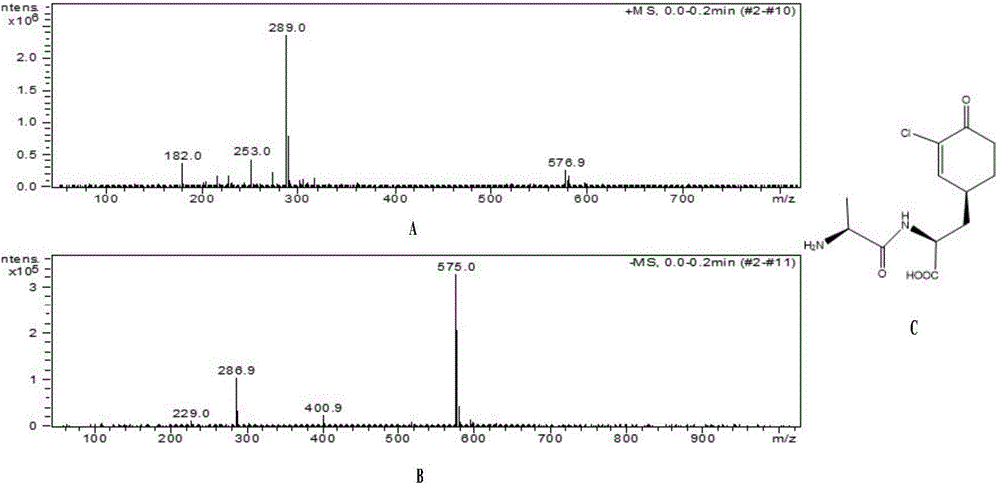
Antibacterial compound and application thereof
A compound, antifungal technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Fermentation of lead compounds
[0040] Bacillus subtilis (Bacillus subtilis) ZJU007 CGMCC NO.4140 was fermented, separated and purified to obtain the lead compound Chlorotetaine, as follows:
[0041] i medium
[0042] (1) Incline medium: LB medium.
[0043] (2) Seed medium (g L -1 ): glucose 15, yeast powder 10, K 2 HPO 4 1. MgSO 4 0.5, FeSO 4 0.01, NaCl 10, add CaCO after adjusting pH 7.0 3 0.4.
[0044] (3) Fermentation medium (g L -1 ): yeast powder 2.72, soluble starch 26.67, (NH 4 ) 2 SO 4 3.95,K 2 HPO 4 2.4, NaCl 10, MgSO 4 0.5, CaCO 3 0.72, CuSO 4 0.02, FeSO 4 0.02, MnSO 4 0.02, pH 6.5.
[0045] (4) Candida albicans medium (g L -1 ): glucose 20, peptone 10, agar 20.
[0046] ii method
[0047] Strain culture conditions Take 1 cycle of activated strains and put them into a seed bottle, fill 100mL medium in a 500mL Erlenmeyer flask with an initial pH value of 7.0, and culture at 200r / min at 30°C for 24h. Then the seed solution i...
Embodiment 2
[0078] Utilize the novel antibacterial compound Chlorotetaine-Lys that embodiment 1 obtains to carry out in vitro antifungal experiment, concrete steps are as follows:
[0079] (1) Five strains of common fungal pathogenic bacteria purchased from China Microbial Culture Collection Center were activated and cultured in vitro; the above five strains of common fungal pathogenic bacteria were respectively Candida albicans (ATCC10231), Aspergillus niger (preserved in the laboratory), Candida parapsilosis (JCM1785), Candida krusei (CBS573), Candida pseudopolymorpha (ATCC 26012)
[0080] The formula of the fermentation medium of Candida and Aspergillus niger is: 4% glucose and 1.0% peptone. The above five common fungal pathogenic bacteria were placed in the above medium at 28° C. and cultured for 18-24 hours.
[0081] (2) Using a 96-well plate, the novel antimicrobial compound is formulated to a final concentration of 100 μg / mL to 0.2 μg / mL by the double dilution method;
[0082] (3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com