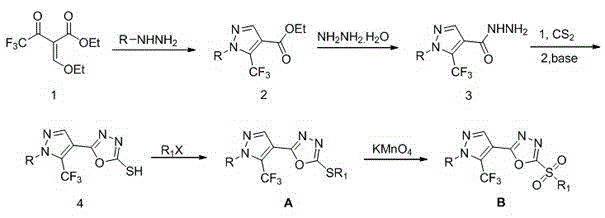
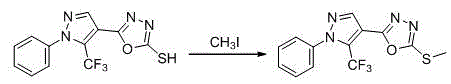1-substituted-5-trifluoromethyl-4-pyrazol-1,3,4-oxadiazole thioether or sulfone derivatives and application of derivatives
A technology of oxadiazole sulfide and trifluoromethyl, which is applied in the field of agricultural plant pathogen prevention and control, and can solve problems such as drug resistance, irrational use, and human health threats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
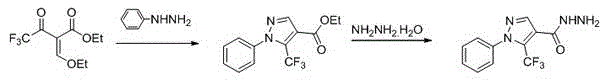
[0038] Example 1: Preparation of 1-phenyl-4-((5-methylthio)-1,3,4-oxadiazolyl)-5-trifluoromethyl-1H-pyrazole (A1)
[0039] 1) 1-Phenyl-5-trifluoromethyl-1 H - Preparation of 4-pyrazole hydrazide
[0040]
[0041] In a 250 mL three-neck flask equipped with a condenser tube and a thermometer, add ethyl ethoxymethylene trifluoroacetoacetate (66.05 mmol), phenylhydrazine (69.35 mmol), and ethanol (50 mL) in sequence, and heat to 80 o C, reacted for 24 h, TLC detected the end of the reaction, lowered to room temperature, distilled off the solvent under reduced pressure, and directly dissolved the remaining oily liquid in 80% hydrazine hydrate solution (100 mmol) without purification, heated to reflux for 2 hours, TLC detected the end of the reaction , the system was lowered to normal temperature, suction filtered, the solid was washed with water and dried to obtain the product, a white solid with a yield of 81.8% and a melting point of 146–148 o C; 1 H-NMR (500 MHz, DMSO-D 6...
Embodiment 2
[0048] Example 2: Preparation of 1-methyl-4-((5-methylthio)-1,3,4-oxadiazolyl)-5-trifluoromethyl-1H-pyrazole (A14)
[0049] 1) Preparation of 1-methyl-5-trifluoromethyl-1H-4-pyrazole hydrazide
[0050]
[0051] In a 250 mL three-necked flask with a condenser tube and a thermometer, add ethyl ethoxymethylene trifluoroacetoacetate (66.05 mmol), methylhydrazine (40%) (69.35 mmol), ethanol (50 mL) in sequence, Heat to 80 o C, reacted for 24 h, TLC detected the end of the reaction, lowered to room temperature, distilled off the solvent under reduced pressure, and directly dissolved the remaining oily liquid in 80% hydrazine hydrate solution (100 mmol) without purification, heated to reflux for 2 hours, TLC detected the end of the reaction , the system was lowered to normal temperature, suction filtered, the solid was washed with water and dried to obtain the product, a white solid with a yield of 64.6% and a melting point of 115-116 o C; 1 H-NMR (500 MHz, DMSO-D 6 ): δ : 9...
Embodiment 3
[0058] Example 3: 1-phenyl-4-((5-ethanesulfonyl)-1,3,4-oxadiazolyl)-5-trifluoromethyl-1 H - Preparation of pyrazole (B1)
[0059]
[0060] In a 25 mL three-neck flask with a condenser tube and a thermometer, add 1-phenyl-4-((5-ethylthio)-1,3,4-oxadiazolyl)-5-trifluoromethyl -1 H -pyrazole (615.8 mu mol), acetic acid (3 mL), and KMnO 4 (615.8 mu mol), react at room temperature for 24 hours, TLC detects the end of the reaction, adjust the pH value to 7~8, filter with suction, and purify the crude product by column chromatography (dichloromethane: ethyl acetate = 10:1), the target compound B1. White solid, yield 64.6%, melting point 72~73 o C; 1 H NMR (500 MHz, DMSO- d6 ) δ : 8.58 (s, 1H, pyrazole H), 7.64-7.58 (m, 5H, benzene H), 3.78 (q, J = 7.3 Hz, 2H, S-CH 2 ), 1.31 (t, J = 7.4 Hz, 3H, CH 3 ); 13 C NMR (125 MHz, DMSO- d 6 ) δ : 161.83, 159.90, 141.92, 138.81, 131.12, 130.02, 126.75, 120.40, 118.24, 107.54, 50.07, 7.15; ν 3089, 2981, 2936, 1627, 1595...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com