Method for simultaneously removing nitrous oxide and nitric oxide in exhaust gas of chemical plant by utilizing catalyst
A technology of nitrous oxide and nitric oxide, which is applied in the fields of nitrous oxide capture, chemical instruments and methods, separation methods, etc., to achieve the effects of simplifying the process, reducing operating costs, and simplifying the waste gas treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
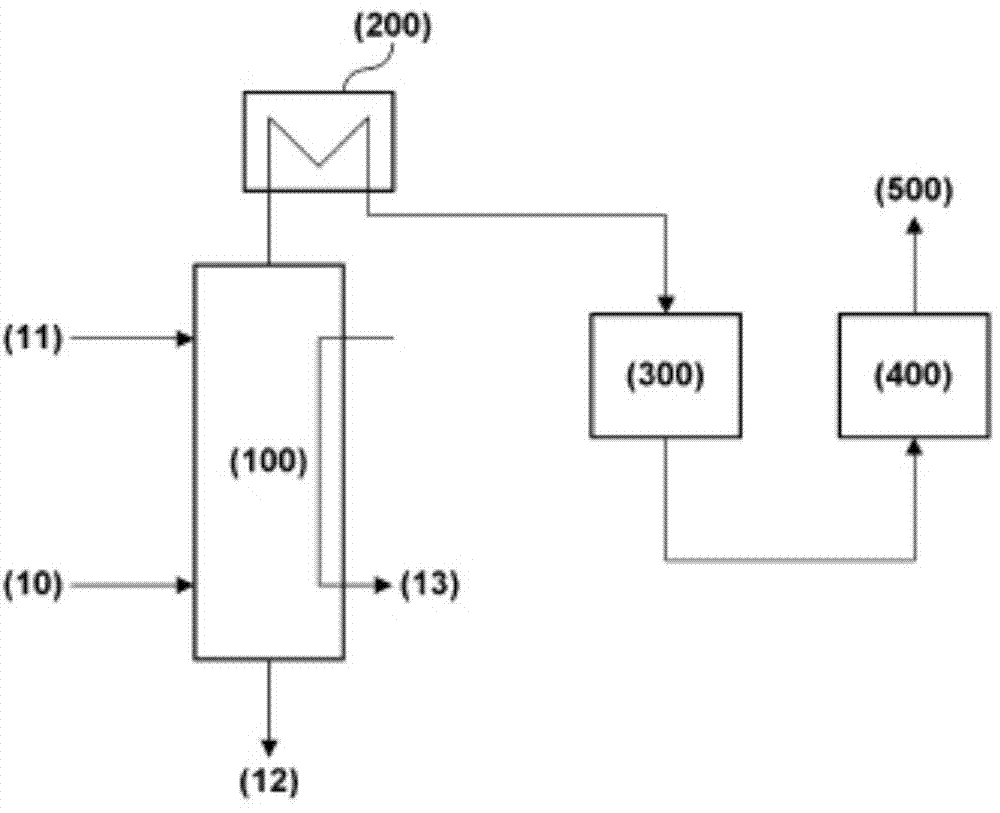
Image
Examples
Embodiment 1
[0050] to Al 2 o 3 / SiO 2 After heating 8 g of BEA zeolite with a molar ratio of 25 to 500°C while supplying 1 liter / min (liter / min) of nitrogen, deionized distilled water was passed through a syringe pump at a flow rate of 0.5 ml / min Serve for 1 hour. After supplying distilled water, the temperature was cooled to normal temperature in the same flow rate of nitrogen to prepare a water-treated BEA zeolite (A).
[0051] Iron ion solution (B) is made by adding 1.6g iron nitrate hydrate (Fe(NO 3 ) 3 9H 2 O) Prepare by dissolving in 1 liter of deionized distilled water. Disperse 8 g of BEA zeolite (A) which has been treated with moisture in 1 liter of iron ion solution (B) prepared and stir at a temperature of 21 for 24 hours. A zeolite filter cake impregnated with iron ions was obtained by filtration of the stirred zeolite slurry, where the filter cake was washed with 1 liter of deionized distilled water. The washed filter cake was dried in air at a temperature of 105° C. ...
Embodiment 2
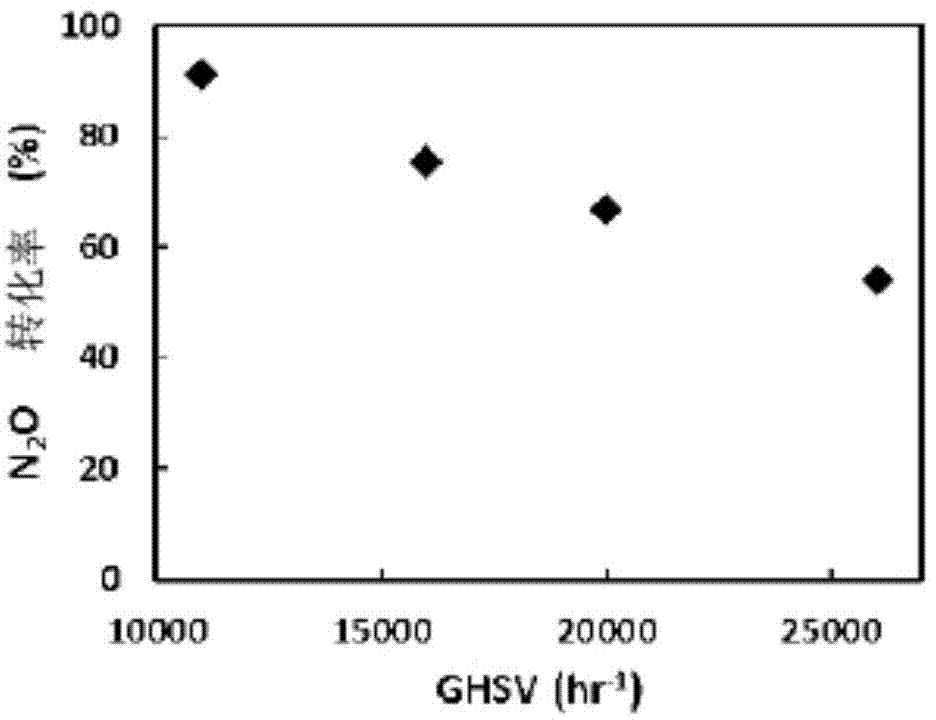
[0057] In order to compare the basic properties of the catalysts produced in the [Example 1] and [Comparative Example], 0.4 g of different catalysts produced were placed in the center of a 1 / 2" stainless steel tube reactor. The temperature of the reactor was raised from room temperature to 500°C at a rate of 4 / min using an electric furnace.
[0058] Nitrous oxide was supplied at a concentration of 400 ppm, and 400 ppm of ammonia reducing agent was used. The oxygen concentration in the reaction gas was adjusted to 3,000 ppm. The total flow of reaction gas was fixed at 0.4 l / min with nitrogen and the space velocity (GHSV) was maintained at 20,000 hr -1 . After the reaction, in order to analyze the components of the gas, an online gas analyzer (SIEMENS) was used for the concentration of nitrous oxide, and the results are shown in the following table.
[0059]
[0060][Example 1] and [Comparative Example] The nitrous oxide conversion rates of the catalysts produced at a reac...
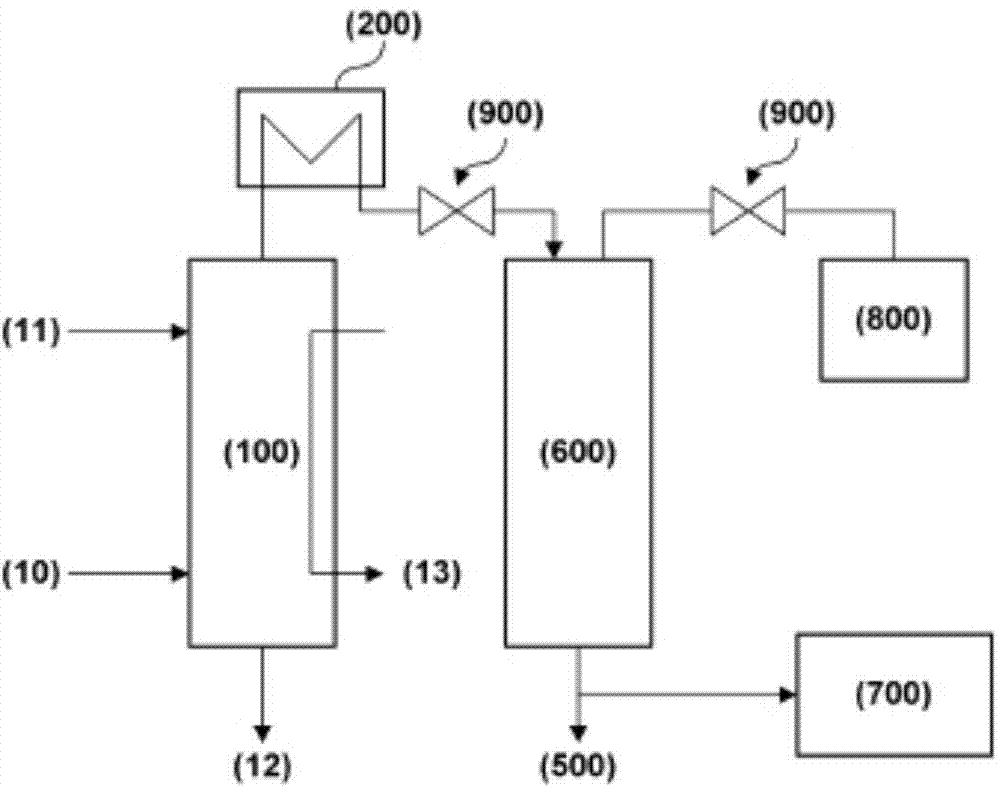
Embodiment 3
[0072] In order to confirm the efficiency of removing nitrous oxide and nitrogen monoxide using the catalyst of the present invention in the waste gas generated in the actual production plant, the waste gas of the nitric acid production plant was used as the raw material gas, and the iron ion impregnated by the present invention Zeolite reactors were used to observe the removal efficiency of nitrous oxide and nitric oxide.
[0073] The zeolite impregnated with iron ions used in this example was produced by the same method as in the above [Example 1]. In order to achieve ion exchange of a sufficient amount of Fe ions and impregnate the zeolite, a total of three times of ion exchange were performed. Impregnation and filtration, washing, drying process. Finally, the dried catalyst was calcined in an atmosphere of 500 for 4 hours.
[0074] Generally, the composition of the exhaust gas emitted from the nitric acid production process is known to be about [NO] hundreds of ppm, [N 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com