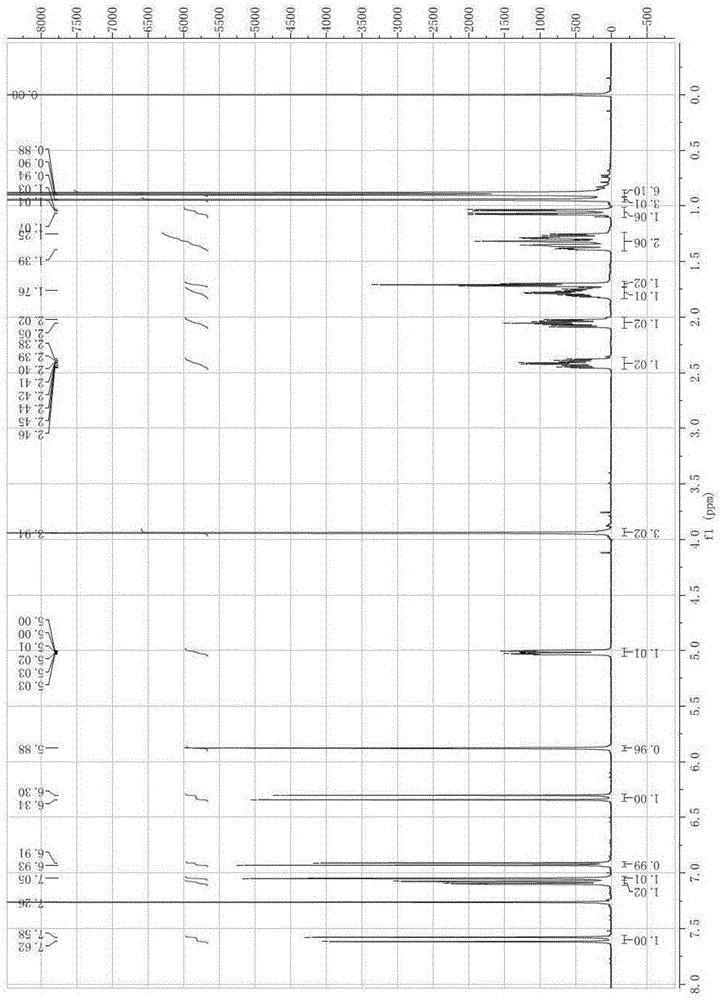
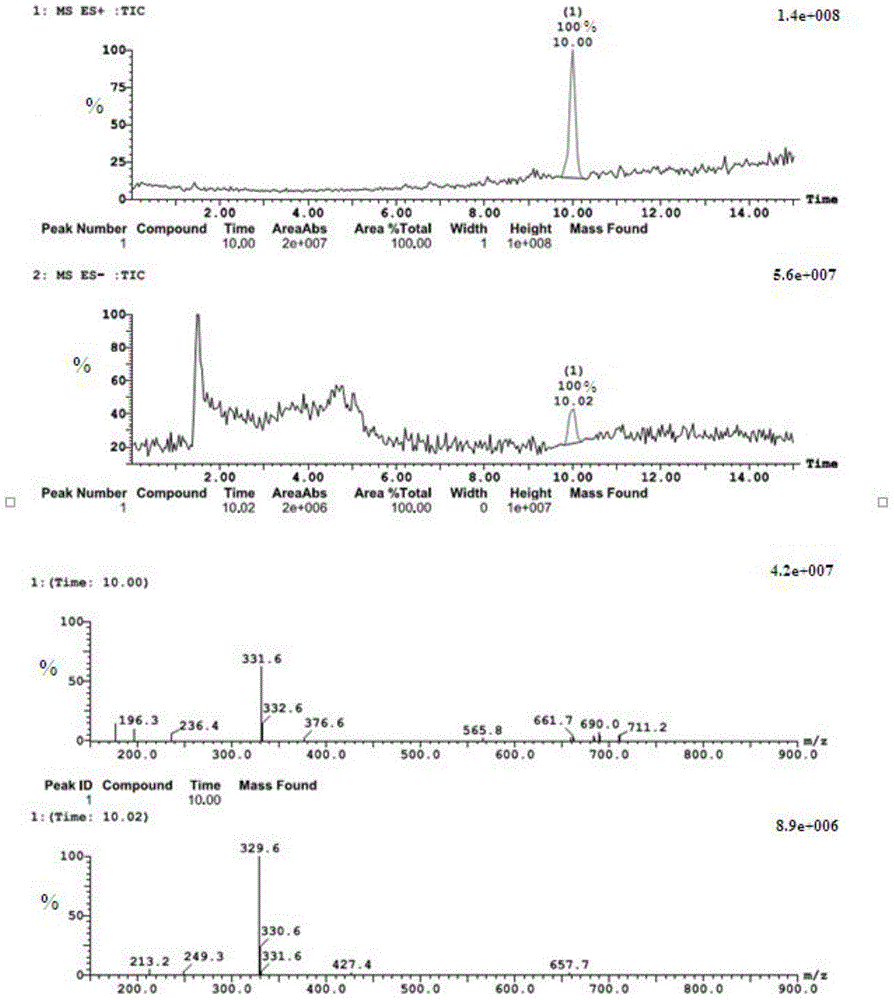
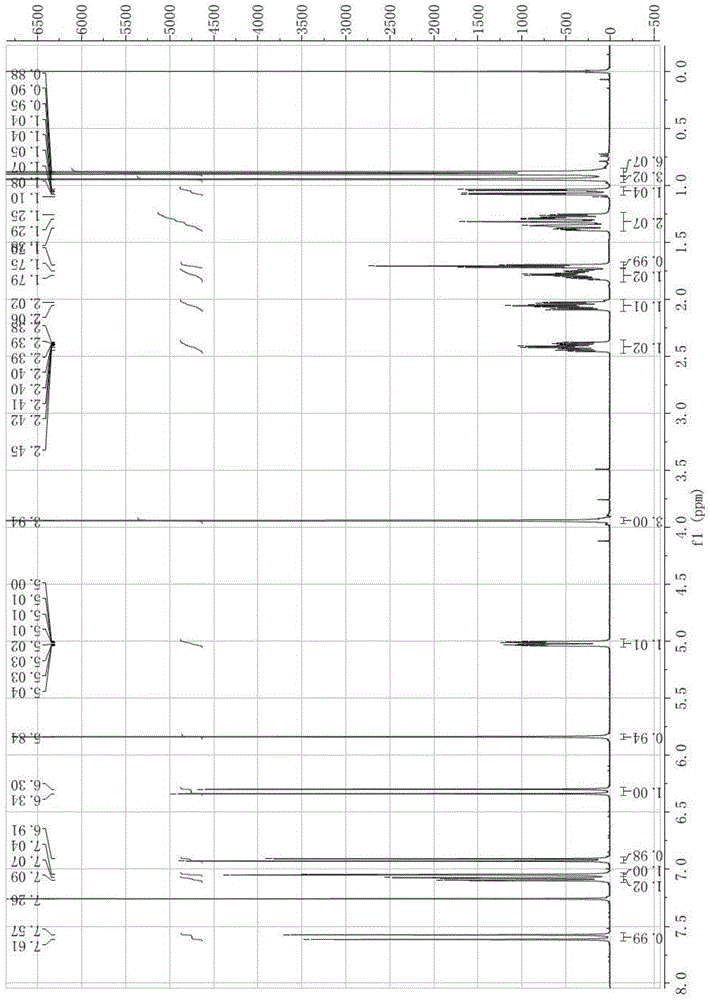
Synthesis and application of bornyl ferulate
A technology of bornyl ferulic acid and ferulic acid, which is applied in the field of chemical medicine, can solve the problems of fast metabolism, strong hydrophilicity, and poor fat solubility, and achieve good fat solubility, easy synthesis process, and high synthesis process. The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A synthetic route and specific synthetic method of D-bornyl ferulate are disclosed in this example, wherein the synthetic route is:
[0042]
[0043] Among them, 1a is dextroborneol, 2 is ferulic acid protected by phenolic hydroxyl group, R is a protecting group, and the ferulic acid protected by phenolic hydroxyl is condensed with dextroborneol under the catalysis of a condensation agent to obtain dextrorotary ferulic acid protected by phenolic hydroxyl group. Bornyl ester 3a, and then deprotected to obtain D-bornyl ferulic acid; the above-mentioned protecting group includes any one of the following: methyl, tert-butyl, triphenylmethyl, methoxymethyl, trimethyl silyl, tert-butyldimethylsilyl, benzyl and tetrahydropyranyl; the above-mentioned condensing agents include: dicyclohexylcarbodiimide, diisopropylcarbodiimide, 1-(3-di Methylaminopropyl)-3-ethylcarbodiimide, 4-dimethylaminopyridine, 1-hydroxybenzotriazole and 4-pyrrolidinylpyridine.
[0044] The synthetic me...
Embodiment 4
[0051] This embodiment discloses a synthetic route of L-bornyl ferulate, and the route is as follows:
[0052]
[0053] Among them, 1b is L-borneol, ferulic acid protected by phenolic hydroxyl group is condensed with L-borneol under the catalysis of a condensing agent to obtain L-bornyl ferulic acid ester 3b protected by phenolic hydroxyl group, and then deprotected to obtain L-bornyl ferulic acid ester.
[0054] The above-mentioned protecting groups include any one of the following: methyl, tert-butyl, triphenylmethyl, methoxymethyl, trimethylsilyl, tert-butyldimethylsilyl, benzyl and tetra Hydropyranyl; Ferulic acid protected by phenolic hydroxyl is condensed with L-borneol under the catalysis of a condensing agent to obtain L-bornyl ferulic acid protected by phenolic hydroxyl, and then deprotected to obtain L-bornyl ferulic acid.
[0055] Specific synthesis method:
[0056] A. Put 4-benzylferulic acid (7.5g, 0.026mol) into dichloromethane (50ml), cool down to 0°C in an ...
Embodiment 5
[0060] A synthetic route of D-bornyl ferulate is disclosed in this example:
[0061]
[0062] Haloacetyl D-bornyl ester 4a is reacted with organophosphorus reagent to obtain wittig phosphorus reagent 5a, and then wittig phosphorus reagent 5a is reacted with 3-methoxy-4 hydroxybenzaldehyde 6 under the action of alkali to obtain ferulic acid dextrose Sporbornyl ester.
[0063] Wherein, X is Cl, Br or I, R is PPh 3 X, P(O)Ph 2 or P(O)(OEt) 2 .
[0064] Organophosphorus reagents include: triphenylphosphine, ethoxydiphenylphosphine and triethyl phosphite; the base includes: lithium aluminum hydride, sodium methoxide, sodium ethoxide, potassium tert-butoxide, sodium hydride, hydrogen Sodium Oxide, Potassium Hydroxide, Lithium Hydroxide and Potassium Carbonate.
[0065] The concrete method step of above-mentioned synthetic route is:
[0066] Step 1: Add chloroacetyl D-bornyl ester 4a (21.5g, 93.2mmol), triphenylphosphine (48.9g, 186mmol), and toluene (215ml) into the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com