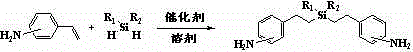
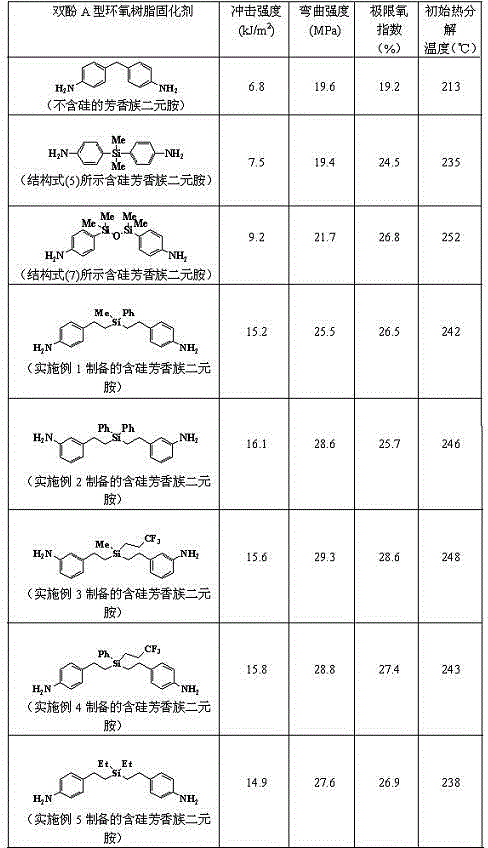
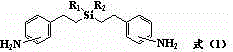
Silicon-containing aromatic diamine and preparation method thereof
An aromatic binary, hydrosilation reaction technology, applied in chemical instruments and methods, compounds of periodic table Group 4/14 elements, organic chemistry, etc., can solve and/or synthetic methods are complicated to operate and difficult to scale industrial Production, three wastes and other serious problems, to achieve the effect of high industrial production value, convenient synthesis of similar compounds, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 The synthesis of methylphenyl bis (4-aminophenethyl) silane
[0029] At 0°C, slowly add a mixture of 12.2g (0.10mol) of methylphenylsilane and 50mL of toluene dropwise to 4mL of 2.0mM isopropanol solution of chloroplatinic acid and 26.2g (0.22mol) of 4-vinylaniline 80mL of toluene solution, stirred at 25°C for 16h, evaporated the solvent under reduced pressure, and recrystallized the residue from butyl acetate to obtain 34.3g of methylphenylbis(4-aminophenethyl)silane as a light off-white solid product. The rate is 95.3%, and the content is 98.4%. Elemental analysis measured value (calculated value) / %: C 76.88 (76.67), H 7.85 (7.78), N 7.91 (7.78). MS (m / z): 361 [M+H] + .
Embodiment 2
[0030] Example 2 Synthesis of diphenyl di(3-aminophenethyl)silane
[0031] At 5°C, a mixture of 18.4g (0.10mol) of diphenylsilane and 50mL of benzene was slowly added dropwise to a 100mL benzene solution containing 1.2g of platinum dioxide and 26.2g (0.22mol) of 3-vinylaniline, The reaction was stirred at 20°C for 20 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl lactate to obtain 39.5 g of off-white solid product diphenylbis(3-aminophenethyl)silane, with a yield of 93.6% and a content of 98.6% . Elemental analysis measured value (calculated value) / %: C 79.77 (79.62), H 7.32 (7.11), N 6.78 (6.64). MS (m / z): 423[M+H] + .
Embodiment 3
[0032] Example 3 Synthesis of methyl (3,3,3-trifluoropropyl) bis (3-aminophenethyl) silane
[0033] At 0°C, a mixture of 14.2 g (0.10 mol) of methyl (3,3,3-trifluoropropyl) silane and 60 mL of dichloromethane was slowly added dropwise to 1,3-divinyl-1,1 , 1.2g of 3,3-tetramethyldisiloxane platinum and 27.4g (0.23mol) of 3-vinylaniline in 80mL of dichloromethane solution, stirred and reacted at 20°C for 15h, evaporated the solvent under reduced pressure, and the residue Recrystallized from dimethyl carbonate to obtain 37.0 g of off-white solid product methyl(3,3,3-trifluoropropyl)bis(3-aminophenethyl)silane, with a yield of 97.3% and a content of 98.2%. Elemental analysis measured value (calculated value) / %: C 62.99 (63.16), H 7.00 (7.11), N 7.56 (7.37). MS (m / z): 381 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com