Paracetamol pseudophedrine sulfateand clemastine fumarate tablets, film-coated tablets and preparation method thereof
A kind of technology of Machuotine tablets and paracetamol, which is applied in the field of pharmaceutical preparations and achieves the effects of simple process and excellent dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 prescription screening
[0026] The main medicines of this product are acetaminophen, pseudoephedrine hydrochloride, and clemastine fumarate. When designing the prescription, the active ingredients are complicated, and the stability and dissolution rate of the preparation are difficult to meet the understanding requirements. Prescription screening is as follows:
[0027] Table 1 Prescription screening (500 tablets dosage)
[0028]
[0029]
[0030] The applicant unexpectedly found in the prescription screening experiment that when glyceryl behenate was added to the prescription and the amount of glyceryl behenate was 1 / 3-1 / 2 of that of magnesium stearate, the drug dissolution rate and stability Significantly improved. In the process step, glyceryl behenate, hydroxypropyl cellulose and magnesium stearate are added simultaneously.
[0031] The test results show that the compressibility of the granules of the five prescriptions is good, and the appear...
Embodiment 2
[0037] Embodiment 2 best prescription and technology
[0038] prescription:
[0039]
[0040]
[0041] Coating layer prescription:
[0042] Gastric Opadry 20.0g
[0043] 75% ethanol 400ml
[0044] Process:
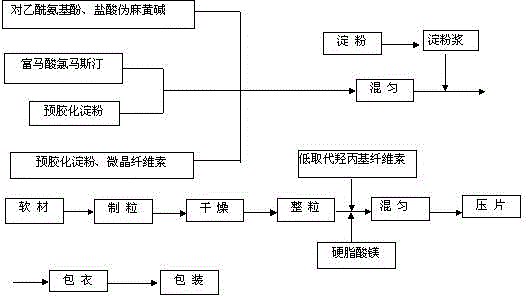
[0045] (1) fully mixing clemastine fumarate with pregelatinized starch in a ratio of 1:100 by weight to obtain clemastine fumarate mother powder for subsequent use;
[0046] (2) adding water to the starch of the prescription amount to make a weight ratio of 6% starch slurry for subsequent use;
[0047] (3) Acetaminophen, pseudoephedrine hydrochloride, clemastine fumarate mother powder, microcrystalline cellulose and the remaining amount of pregelatinized starch are weighed and mixed thoroughly;
[0048] (4) Add starch slurry to make soft material, and granulate with 14 mesh sieves;
[0049] (5) Blast drying at 60°C, granulation with a 16-mesh sieve;
[0050] (6) Add hydroxypropyl cellulose and magnesium stearate of prescription quantity, fully mix;
[0051] (...
Embodiment 3
[0055] Experimental data and literature data of embodiment 3 drug stability research
[0056] 1. Sample source and batch number
[0057] Sample: Prepared by Jinan Limin Pharmaceutical Co., Ltd. according to the prescription and process of Example 2.
[0058] Batch numbers: 1401104, 1401105, 1401106.
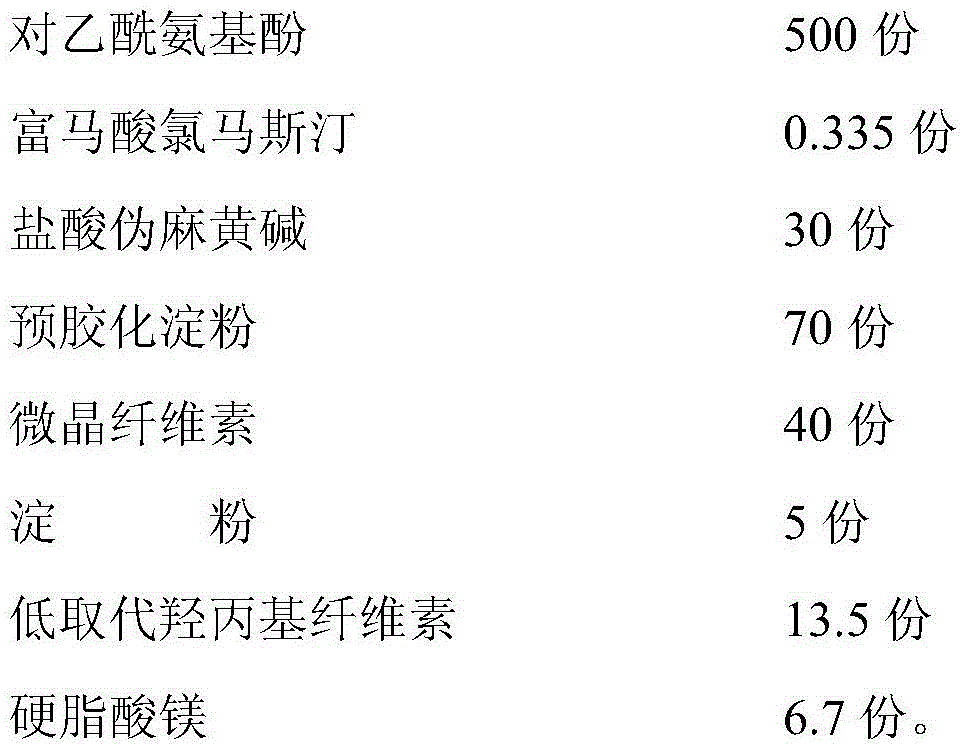
[0059]Specifications: Acetaminophen 500mg, Pseudoephedrine Hydrochloride 30mg, Clemastine Fumarate 0.335mg.
[0060] Quantity: 10,000 pieces per batch.
[0061] 2. Inspection items and testing methods
[0062] Properties, dissolution rate, related substances, content. The above items are determined by the methods in the quality standards except for the "related substances" which are determined by the related substances determination method in the quality research.
[0063] 3. Experimental research
[0064] (1) Influencing factor test
[0065] Light test:
[0066] Get this product (batch number: 1401104) and set the light intensity 4500LX to irradiate for 10 days, take sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com