Preparation method of novel flower-like basic copper chloride catalyst
A copper chloride and catalyst technology, applied in the field of preparation of novel flower-shaped basic cupric chloride catalysts, can solve the problems of large particle size, uneven morphology and the like, and achieve efficient degradation, stable existence, and simple and feasible methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of flower shape basic copper chloride catalyst
[0024] (1) Preparation of aqueous sodium hydroxide solution: Add 2 g of sodium hydroxide to 100 mL of water to obtain a 0.5 mol / L sodium hydroxide solution;
[0025] (2) Dissolving at room temperature: Take 12.5ml of sodium hydroxide aqueous solution, add 0.682g of copper chloride dihydrate and 12.5ml of 30% hydrogen peroxide, stir at room temperature to dissolve copper chloride dihydrate, and make the concentration of copper chloride 0.16mol / L mixed solution;
[0026] (3) Hydrothermal reaction: Add the above mixed solution into the polytetrafluoroethylene lining, conduct a hydrothermal reaction at a constant temperature of 120°C for 20 hours, centrifuge at 3000 rpm for 3 minutes, wash with distilled water and absolute ethanol in turn, and dry at 60°C for 4 hours , That is, flower-shaped basic copper chloride catalyst.
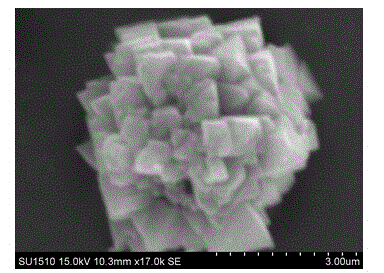
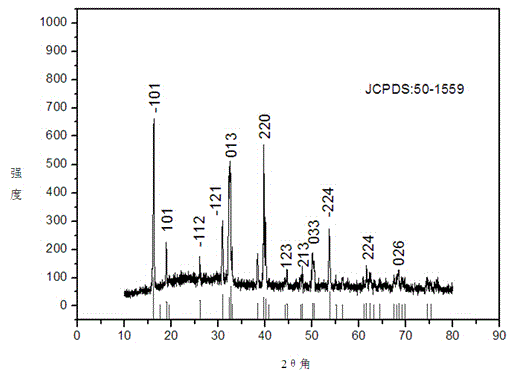
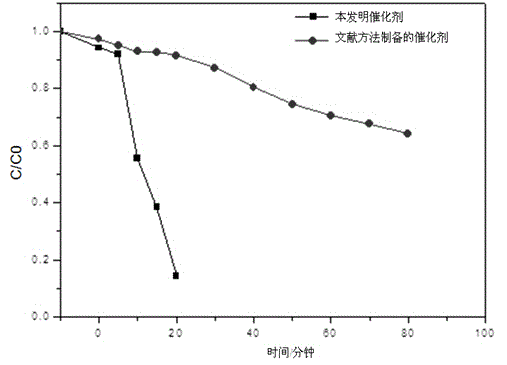
[0027] Depend on figure 2 It can be seen that the prepared catalys...
Embodiment 2
[0028] Embodiment 2: the preparation of flower shape basic copper chloride catalyst
[0029] (1) Preparation of sodium hydroxide aqueous solution: add 1.28g sodium hydroxide to 100mL water to obtain 0.32mol / L sodium hydroxide solution;
[0030] (2) Dissolving at room temperature: Take 20ml of sodium hydroxide aqueous solution, add 0.656g of copper chloride dihydrate and 5ml of 30% hydrogen peroxide, stir at room temperature to dissolve copper chloride dihydrate, and make a mixture with a concentration of copper chloride of 0.154mol / L solution;
[0031] (3) Hydrothermal reaction: Add the above mixed solution into the polytetrafluoroethylene lining, conduct a hydrothermal reaction at a constant temperature of 80°C for 24 hours, centrifuge at 3000 rpm for 3min, wash with distilled water and absolute ethanol in turn, and dry at 60°C for 4h , That is, flower-shaped basic copper chloride catalyst.
[0032] The composition of the prepared catalyst was consistent with that of Exam...
Embodiment 3
[0033] Embodiment 3: the preparation of flower shape basic copper chloride catalyst
[0034] (1) Preparation of sodium hydroxide aqueous solution: add 3.2g sodium hydroxide to 100mL water to obtain 0.8mol / L sodium hydroxide solution;
[0035] (2) Dissolving at room temperature: Take 24ml of sodium hydroxide aqueous solution, add 0.699g of copper chloride dihydrate and 1ml of 30% hydrogen peroxide, stir at room temperature to dissolve copper chloride dihydrate, and make a mixture with a concentration of copper chloride of 0.164mol / L solution;
[0036] (3) Hydrothermal reaction: Add the above mixed solution into the polytetrafluoroethylene lining, conduct a hydrothermal reaction at a constant temperature of 140°C for 18 hours, centrifuge at 3000 rpm for 3 minutes, wash with distilled water and absolute ethanol in turn, and dry at 60°C for 4 hours , That is, flower-shaped basic copper chloride catalyst.
[0037] The composition of the prepared catalyst was consistent with tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com