Pentaerythritol tetraacrylate preparation method
A technology of pentaerythritol acrylate and pentaerythritol, which is applied in the field of esterification, can solve the problems of poor decolorization effect of pentaerythritol acrylate, and achieve the effect of obvious decolorization effect, simple operation and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] As attached figure 1 As shown, the embodiment of the present invention provides a method for preparing pentaerythritol acrylate, the method including:
[0029] Step 101: Add pentaerythritol, acrylic acid, solvent, catalyst and first polymerization inhibitor to the reactor, and heat the reactor to a preset temperature.
[0030] Step 102: Make the reaction system reflux and react at the preset temperature, and track the molar ratio of pentaerythritol triacrylate to pentaerythritol tetraacrylate in the reaction system, when the molar ratio of pentaerythritol triacrylate to pentaerythritol tetraacrylate is 1:3- At 10 o'clock, the reflux reaction was ended.
[0031] Step 103: After the reaction system is cooled, the reaction system is washed with water and alkali washed, and the first organic phase is taken.
[0032] Step 104: Wash the first organic phase with a reducing agent, then wash the first organic phase with water until it is neutral, and take the second organic phase.
[003...
Embodiment 2
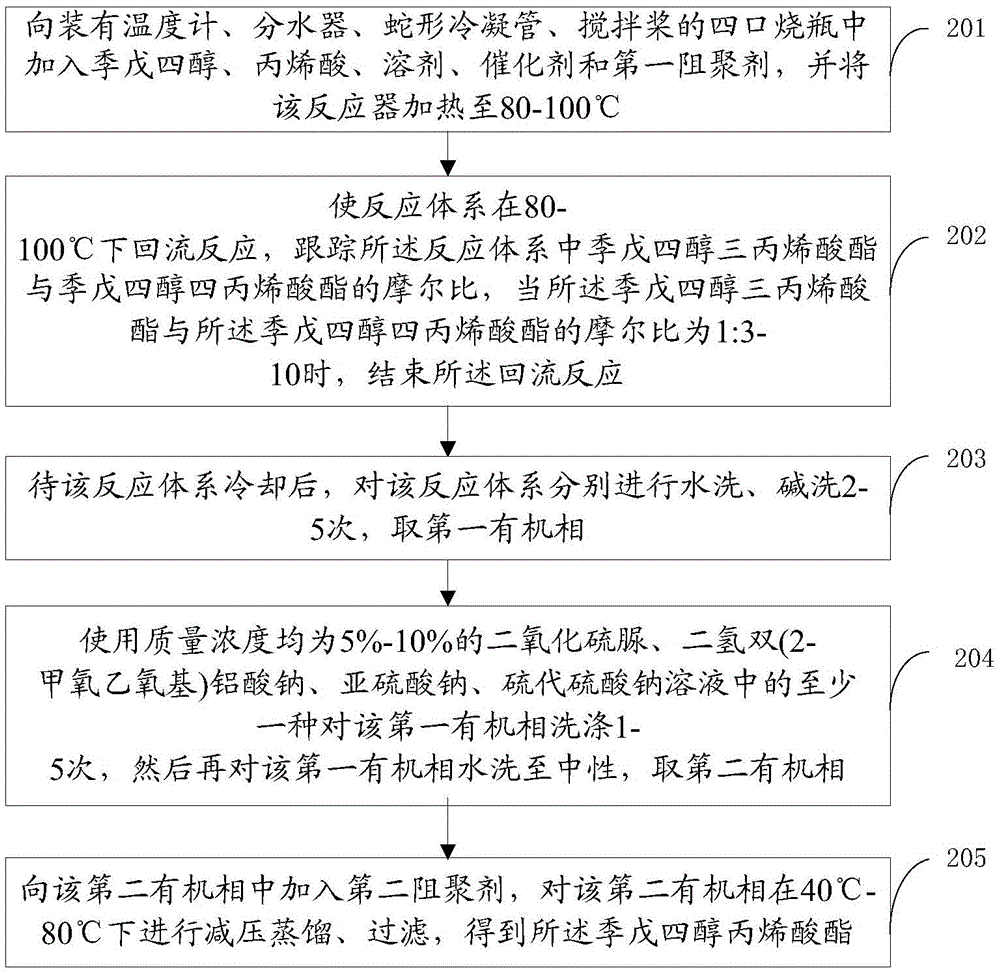
[0036] As attached figure 2 As shown, the embodiment of the present invention provides a method for preparing pentaerythritol acrylate, the method including:
[0037] Step 201: Add pentaerythritol, acrylic acid, solvent, catalyst and first polymerization inhibitor to a four-necked flask equipped with thermometer, water trap, serpentine condenser and stirring blade, and heat the reactor to 80-100°C .
[0038] Specifically, the molar ratio of pentaerythritol and acrylic acid is 1:4-8, preferably 1:4-6.5. In the embodiment of the present invention, to ensure that the molar ratio of pentaerythritol triacrylate to pentaerythritol tetraacrylate is 1:3-10, and to ensure a higher conversion rate of pentaerythritol and acrylic acid, the molar ratio of pentaerythritol to acrylic acid is determined to be 1:4 -8, preferably 1:4-6.5. The desired low hydroxyl value pentaerythritol acrylate is prepared by the above-mentioned ratio of pentaerythritol and acrylic acid.
[0039] Wherein, the firs...
Embodiment 3
[0054] 28.87g pentaerythritol, 61.13g acrylic acid, 49.5g toluene, 48.7g cyclohexane, 1.35g methanesulfonic acid, 1.35g HND-6 solid super acid, 0.3g copper sulfate pentahydrate, 0.045g p-hydroxyanisole Put it into a 500ml four-necked flask equipped with a thermometer, a water trap, a serpentine condenser and a stirring blade, and add 13.2 g of toluene and 12.4 g of cyclohexane into the water trap.
[0055] Turn on the oil bath heating device, heat the reaction system to a temperature of 90°C, and reflux for 15 hours. When the ratio of pentaerythritol triacrylate to pentaerythritol tetraacrylate in the reaction system reaches 1:3, the reflux reaction is terminated by high performance liquid chromatography.
[0056] Cool the reaction system down to 35°C, then wash the reaction system twice with a 10% sodium chloride solution, stand for layering, take the upper organic phase to determine the acid value; use an excess of 10% by weight according to the measured acid value 10% sodium hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com