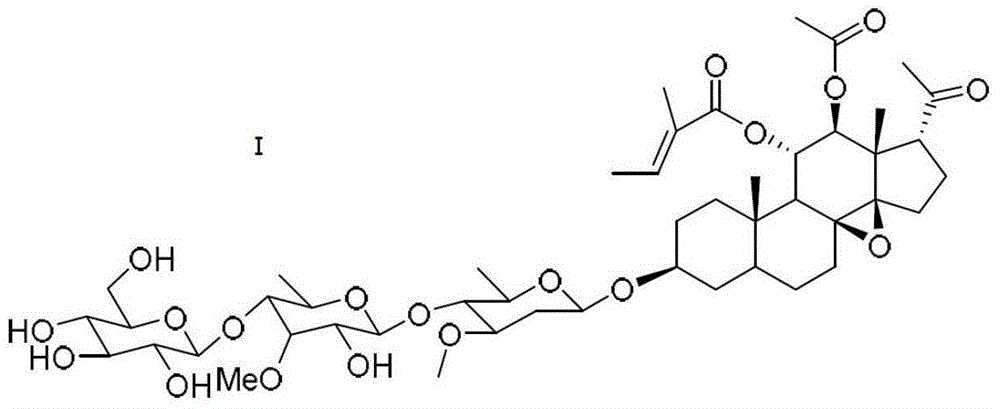
C21 steroid sapogenin derivative of dihydro parazole piperazidine, and preparation method and application thereof
A technology of saponin and derivatives, applied in steroids, drug combinations, antitumor drugs, etc., can solve the problems of insufficient research and achieve the effect of mild experimental environment, high selectivity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
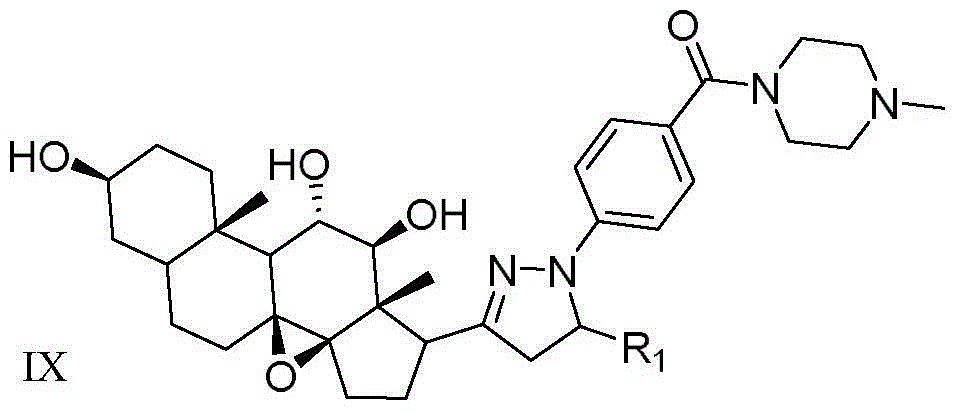
[0066] N-methyl-4-(3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxyl)-(10a,12a-dimethyl)-(1 ,2-cyclopentyl)-1,10a-b-epoxy tetradecyl)-(4,5-dihydropyrazole)) preparation of benzoylpiperazine (compound 18)
[0067]
[0068] Under stirring at -20°C, add the corresponding intermediate 18 (10.0mmol) and dichloromethane (25mL) obtained in step 7 to a 50mL round bottom flask in turn, and gradually add boron tribromide (5.0mmol) dropwise and continue stirring After reacting for 1 h, the reaction flask was transferred to room temperature, and the reaction was continued for 12 h. TLC tracking reaction (developing agent V AcOEt :V 正己烷 =1:2), after the reaction was completed, filtered, the solid was washed with distilled water, and finally dried in vacuum, the obtained solid was dissolved in absolute ethanol, purified by recrystallization, and the target compound 22 was obtained as crystals.
[0069] White crystals were obtained with a yield of 81.6%. m.p.198~199℃; 1 H NMR (D...
Embodiment 2
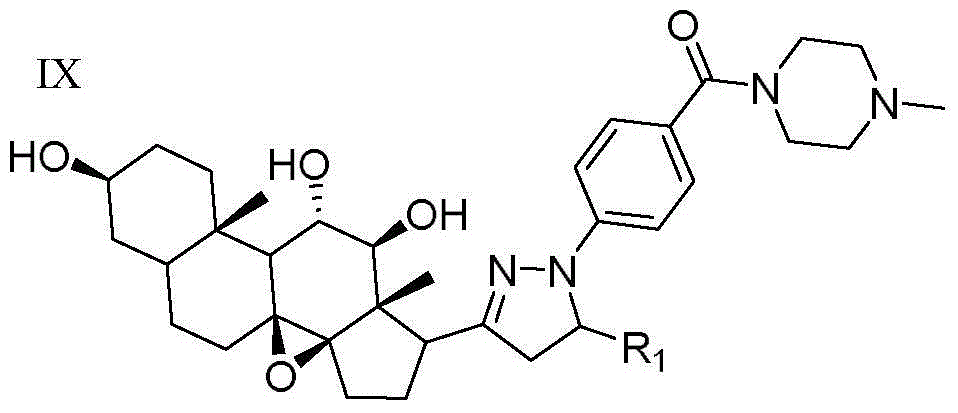
[0071] N-methyl-4-(5-methyl-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy)-(10a,12a-dimethyl Base)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole))benzoylpiperazine (compound 23) preparation of
[0072]
[0073] The preparation method refers to Example 1.
[0074] White crystals were obtained with a yield of 77.6%. m.p.203~205℃; 1 H NMR (DMSO-d 6 ,300MHz)δ:8.00(d,J=8.1Hz,2H,ArH),7.69(d,J=7.7Hz,2H,ArH),5.37(s,2H,OH),4.49(s,1H,OH) ,3.58~3.33(m,7H,CH and CH 2 ),2.70~2.66(m,1H,CH),2.38~2.31(m,6H,CH 2 ),2.18(s,3H,CH 3 ), 2.01(t, J=7.3Hz, 1H, CH), 1.79~1.21(m, 18H, CH, CH 2 and CH 3 ),1.14(dd,J 1 =7.1Hz,J 2 =7.8Hz,1H,CH),0.88(s,3H,CH 3 ),0.81(s,3H,CH 3 ).ESI-MS:593.8[M+H] + .Anal.Calcd for C 35 h 50 N 4 o 5 .
Embodiment 3
[0076] N-methyl-4-(5-ethyl-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy)-(10a,12a-dimethyl Base)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole))benzoylpiperazine (compound 24) preparation.
[0077]
[0078] The preparation method refers to Example 1.
[0079]White crystals were obtained with a yield of 83.9%. m.p.211~212℃; 1 H NMR (DMSO-d 6 ,300MHz)δ:7.97(d,J=8.1Hz,2H,ArH),7.68(d,J=7.7Hz,2H,ArH),5.37(s,2H,OH),4.49(s,1H,OH) ,3.56~3.34(m,7H,CH and CH 2 ),2.71~2.68(m,1H,CH),2.38~2.31(m,6H,CH 2 ),2.18(s,3H,CH 3 ), 2.01(t, J=7.3Hz, 1H, CH), 1.79~1.21(m, 17H, CH and CH 2 ),1.14(dd,J 1 =7.1Hz,J 2 =7.8Hz,1H,CH),0.89(s,3H,CH 3 ),0.87(s,3H,CH 3 ),0.83(s,3H,CH 3 ).ESI-MS:621.8[M+H] + .Anal.Calcd for C 36 h 52 N 4 o 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com