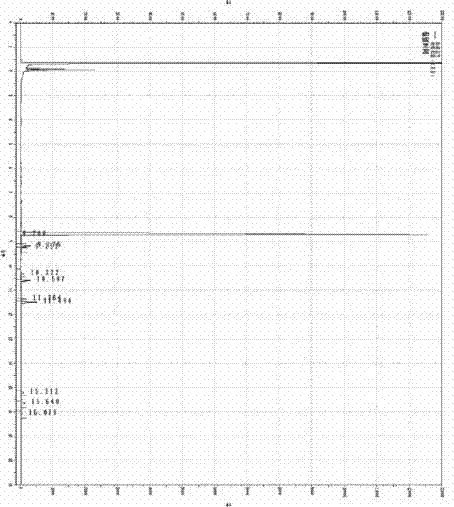
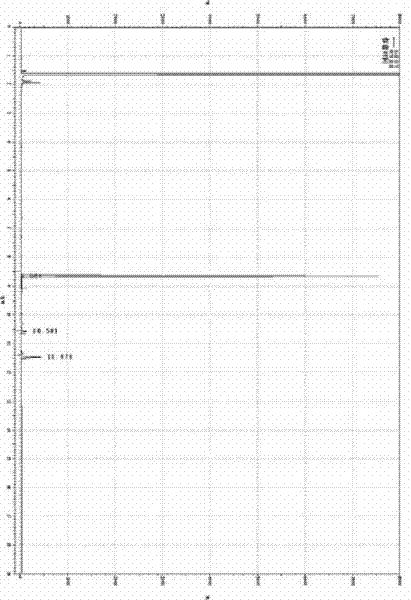
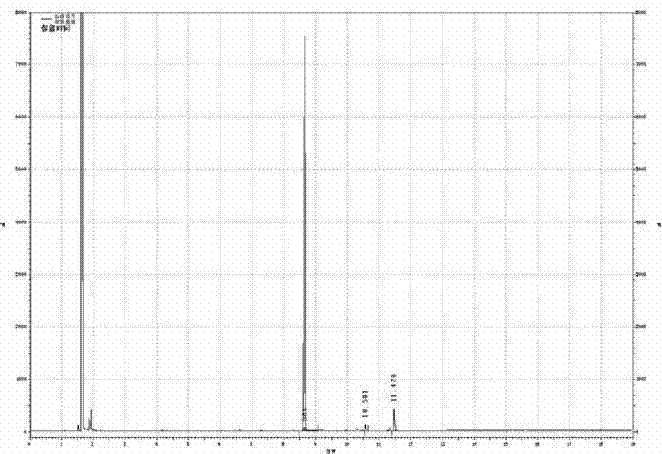
Synthesis method of glycal
A synthesis method and technology of alkenose, applied in the direction of organic chemistry, etc., to achieve the effects of high yield, high product purity and fixed reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthetic method of diacetyl ribosene:
[0029] (1) Take 1000ml of dry and clean one-mouth bottle, add 150g of dichloromethane, and then add 100g of pure triacetyl ribose bromide to dissolve triacetyl ribose bromide to form a solution, and cool to below 10 degrees;
[0030] (2) Take 1000ml of dry and clean three-neck round-bottom flask, install a stirring and condenser, then add 150g of dichloromethane, 50g of zinc powder and 100g of 25wt% ammonium chloride aqueous solution, and mix well;
[0031] (3) Then start the stirring of the three-necked round-bottom flask, the rotating speed is 100r / min, heat the mixture to reflux, stop heating, and start adding the solution prepared in step (1) in batches, adding 20% of the mass of the bromide solution each time One-third, after each addition, due to the exothermic reaction, reflux will occur in the flask. When the reflux phenomenon stops, add the solution again. After all the solution is added, the reflux stops, and the enti...
Embodiment 2
[0035] Synthetic method of diacetyl ribosene:
[0036] (1) Take a 1000ml dry and clean one-mouth bottle, add 150g of dichloromethane, and then add 100g of triacetyl ribose bromide to dissolve the triacetyl ribose bromide to form a solution, and cool to below 10°C;
[0037] (2) Take 1000ml of dry and clean three-necked round-bottom flask, install a stirring and condenser, then add 150g of dichloromethane, 50g of zinc powder and 100g of 30wt% ammonium chloride aqueous solution, and mix well;
[0038] (3) Then start the stirring of the three-necked round-bottom flask, the rotating speed is 100r / min, heat the mixture to reflux, stop heating, and start adding the solution prepared in step (1) in batches, adding 20% of the mass of the bromide solution each time One-third, after each addition, due to the exothermic reaction, reflux will occur in the flask. When the reflux phenomenon stops, add the solution again. After all the solution is added, the reflux stops, and the entire syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com