Stilbene coumarin derivative as well as preparation method and application thereof
A technology of stilbene and coumarin, which is applied in the field of novel stilbene coumarin derivatives and preparation, can solve the problems of poor selectivity, single structure, low bioavailability, etc., and achieves compound structure optimization and reaction The effect of high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
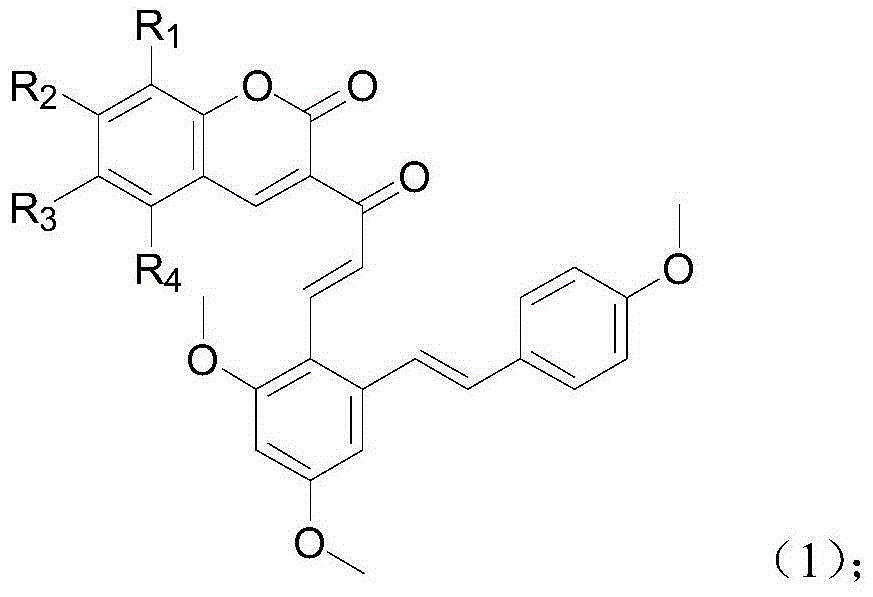
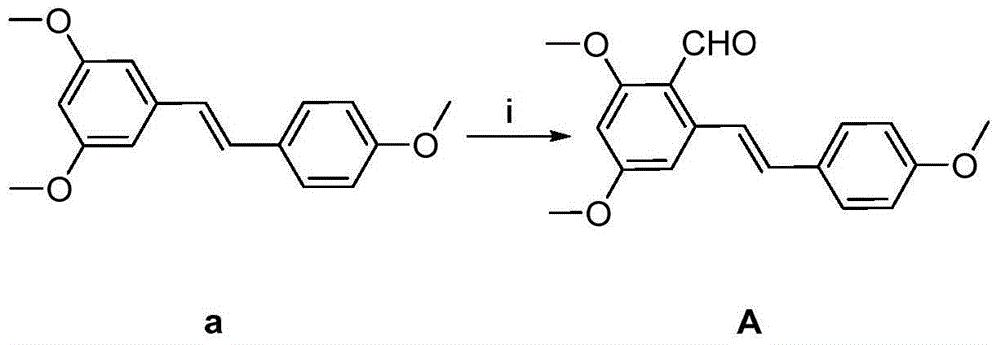
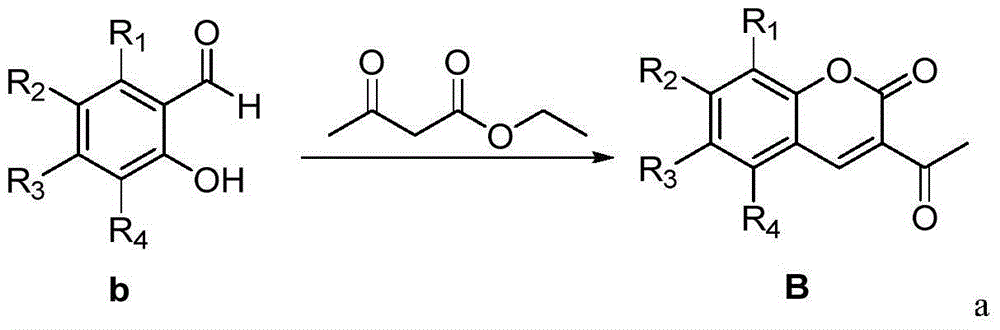
[0030] Example 1: 3-((E)-3-(2,4-dimethoxy-6-(4-methoxystyryl)phenyl)acryloyl)-2H-pyran-2-one (Compound 1) Preparation
[0031]
[0032] (1) Take a 100mL round-bottomed flask, add N,N-dimethylformamide (30mL) in an ice-water bath, and weigh resveratrol trimethyl ether (13.5g, 0.075mol), add 10mL N,N- Dimethylformamide was dissolved, and then added to a round bottom flask, and phosphorus oxychloride (7 mL, 0.075 mol) was slowly added dropwise. After the dropwise addition, the solution was returned to room temperature for reaction, and stirred for 1 h. After the reaction, take a 1000mL beaker, add 500mL ice water and 100mL ethyl acetate, then add the reaction solution dropwise, add solid sodium carbonate in portions under stirring until no bubbles are generated, and precipitate a light yellow solid overnight, filter and dry , column chromatography to obtain (E)-2,4-dimethoxy-6-(4-methoxystyrene)benzaldehyde (compound A). The product was a yellow solid with a yield of 92.7% a...
Embodiment 2
[0035] Example 2: 3-((E)-3-(2,4-dimethoxy-6-(4-methoxystyryl)phenyl)acryloyl)-8-methoxy-2H-benzene Preparation of pyran-2-one (compound 2)
[0036]
[0037] The preparation method is the same as in Example 1. The difference is that 3-methoxy salicylaldehyde is used instead of salicylaldehyde to obtain the target compound as a yellow-brown solid powder with a yield of 63% and a melting point of 115-116°C. 1 H NMR (600MHz, CDCl 3 )δ8.44(s,1H),8.23(d,J=15.7Hz,1H),7.92(d,J=15.7Hz,1H),7.49(d,J=8.7Hz,2H),7.38(d, J=16.0Hz, 1H), 7.22(d, J=7.9Hz, 1H), 7.18(dd, J=7.8, 1.3Hz, 1H), 7.13(dd, J=8.0, 1.2Hz, 1H), 6.91( dd,J=17.3,12.4Hz,3H),6.71(d,J=2.3Hz,1H),6.41(d,J=2.3Hz,1H),3.96(s,3H),3.91(s,3H), 3.88(s,3H),3.83(s,3H). 13 C NMR (151MHz, cdcl 3 )δ190.23(s),164.60(s),164.03(s),162.26(s),161.19(s),149.77(s),149.68(s),147.46(s),145.20(s),141.81( s), 135.04(s), 132.52(s), 130.89(s), 129.21(s), 129.07(s), 127.55(s), 127.19(s), 123.61(s), 121.91(s), 118.43( s),117.99(s),116.81(s),106.3...
Embodiment 3
[0038] Example 3: 3-((E)-3-(2,4-dimethoxy-6-(4-methoxystyryl)phenyl)acryloyl)-7-methoxy-2H-benzene Preparation of pyran-2-one (compound 3)
[0039]
[0040] The preparation method was the same as in Example 1, except that 4-methoxy salicylaldehyde was used instead of salicylaldehyde to obtain the target compound as a yellow solid powder with a yield of 69% and a melting point of 137-139°C. 1 H NMR (600MHz, DMSO) δ8.59(s,1H),8.19(s,1H),8.02–7.95(m,2H),7.82(d,J=8.7Hz,1H),7.68–7.61(m, 2H), 7.53(d, J=8.7Hz, 2H), 7.33(d, J=16.1Hz, 1H), 7.22(d, J=8.7Hz, 1H), 7.08(d, J=16.1Hz, 1H) ,6.90(d,J=8.7Hz,2H),3.88–3.84(m,9H),3.75(s,3H). 13 C NMR (151MHz, DMSO) δ190.16(s), 164.61(s), 164.02(s), 162.26(s), 161.96(s), 158.88(s), 152.32(s), 149.44(s), 145.20 (s), 141.83(s), 135.02(s), 132.49(s), 131.35(s), 129.19(s), 129.10(s), 127.59(s), 124.77(s), 121.57(s), 120.34 (s),117.23(s),116.79(s),113.47(s),106.40(s),100.21(s),59.63(s),59.35(s),59.07(s),58.25(s).MS (EI):469.17(C 30 h 26 o 7 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com