Preparation method and application of a kind of thiol/disulfide bond controllable self-crosslinking hyaluronic acid hydrogel
A hyaluronic acid and hydrogel technology, applied in medical science, prosthesis, etc., can solve the problems affecting the biocompatibility of hydrogel materials, achieve good water solubility, convenient gel method, and controllable physical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
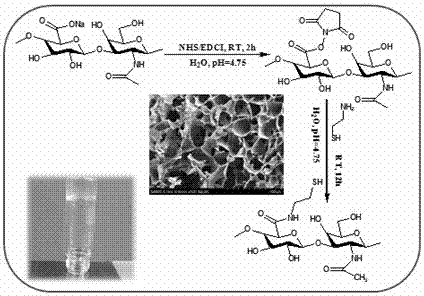
[0061] (1) Dissolve 400mg of sodium hyaluronate in 100mL of deionized water, first add 230mg of N-succinimide (NHS), fully dissolve; then add 385mg of 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (EDC·HCl) powder, adjust the pH=4.75 of the reaction solution with 1M NaOH and 1M HCl solution, and react for 2 hours; then add 10mL of 11.36mg / mL cysteine ( CSH·HCl) hydrochloride, react for 24 hours; finally adjust the pH of the reaction solution to 8.5 with 1M NaOH solution, add 10 mL of 46.5 mg / ml dithiothreitol (DTT) solution, and react for 12 hours.
[0062] (2) After the reaction, adjust the pH of the reaction solution to 3.0-3.5 with 1M HCl solution, dialyze in deionized water with pH=3.0-3.5 for 72 hours, and freeze-dry to obtain cysteine-modified hyaluronic acid.
[0063] Among them, the molecular weight of sodium hyaluronate is 0.1MDa, carboxyl group in sodium hyaluronate: N-succinimide: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride: half The m...
Embodiment 2
[0067] (1) Dissolve 400mg of sodium hyaluronate in 100mL of deionized water, first add 230mg of N-succinimide (NHS), fully dissolve; then add 575mg of 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (EDC·HCl) powder, adjust the pH=4.75 of the reaction solution with 1M NaOH and 1M HCl solution, and react for 2 hours; then add 10mL of 22.72mg / mL cysteine ( CSH·HCl) hydrochloride, react for 24 hours; finally adjust the pH of the reaction solution to 8.5 with 1M NaOH solution, add 10 mL of 93.0 mg / ml dithiothreitol (DTT) solution, and react for 12 hours.
[0068] (2) After the reaction, adjust the pH of the reaction solution to 3.0-3.5 with 1M HCl solution, dialyze in deionized water with pH=3.0-3.5 for 72 hours, and freeze-dry to obtain cysteine-modified hyaluronic acid.
[0069] Among them, the molecular weight of sodium hyaluronate is 0.1MDa, carboxyl group in sodium hyaluronate: N-succinimide: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride: half The m...
Embodiment 3
[0073](1) Dissolve 400mg sodium hyaluronate in 100mL deionized water, first add 230mg N-succinimide (NHS), fully dissolve; then add 770mg 1-ethyl-(3-dimethylaminopropyl) Carbodiimide hydrochloride (EDC·HCl) powder, adjust the pH=4.75 of the reaction solution with 1M NaOH and 1M HCl solution, and react for 2 hours; then add 10mL45.44mg / mL of cysteine ( CSH·HCl) hydrochloride, react for 24 hours; finally adjust the pH of the reaction solution to 8.5 with 1M NaOH solution, add 10 mL of 186.0 mg / ml dithiothreitol (DTT) solution, and react for 12 hours.
[0074] (2) After the reaction, adjust the pH of the reaction solution to 3.0-3.5 with 1M HCl solution, dialyze in deionized water with pH=3.0-3.5 for 72 hours, and freeze-dry to obtain cysteine-modified hyaluronic acid.
[0075] Among them, the molecular weight of sodium hyaluronate is 0.1MDa, carboxyl group in sodium hyaluronate: N-succinimide: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride: half The mass ratio of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
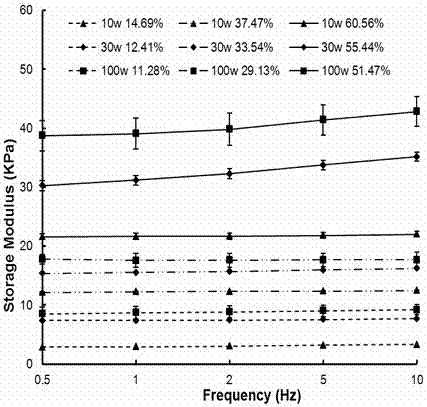
| storage modulus | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com