Method for preparing nanometer SnO2 through self-propagation high-temperature synthesizing technology
A self-propagating high-temperature, nanotechnology, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of affecting the reaction effect, easy moisture absorption during storage, and low product yield. Extended spraying time, simple and easy operation process, promotes fully completed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
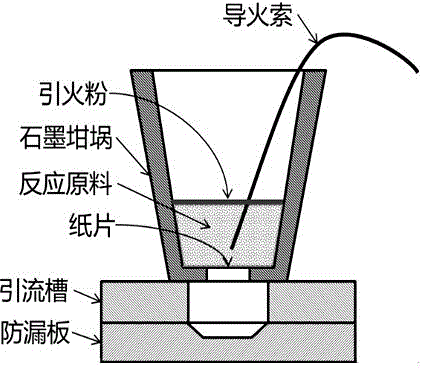
[0030] Mix 45.5% Al+CuO (strictly in accordance with the reaction ratio, that is, the molar ratio is 2:3) and 54.5% Sn powder, stir evenly, put figure 1 Inside the graphite crucible shown. Then sprinkle the ignition powder on the surface of the powder, insert the fuse to ignite the reaction. After the reaction is completed, the product is completely collected in a pre-placed collection device.
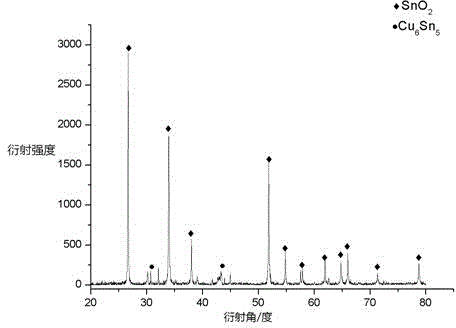
[0031] The collected product was weighed, and the output was 5.5g, and then the prepared product was observed with a field emission scanning electron microscope, as shown in the photo figure 2 shown. It can be seen from the figure that the morphology of the product is linear, and the diameter distribution range is narrow, concentrated around 85nm, that is, nanowires. Finally, the product was also analyzed by X-ray diffraction, and the analysis results were as follows: image 3 shown. It can be seen from the diffraction peaks in the figure that the crystallization of the product i...
Embodiment 2
[0033] The mass fractions are 44% Al+CuO (strictly according to the reaction ratio, that is, the molar ratio is 2:3), 52% Sn powder and 4% CaF 2 Mix the powder, stir well, put in figure 1 Inside the graphite crucible shown. Then sprinkle the ignition powder on the surface of the powder, insert the fuse to ignite the reaction. After the reaction is completed, the product is completely collected in a pre-placed collection device.
[0034] The collected product is weighed, and the output is 7g, which is the same as that without adding CaF 2 Compared with the production increased by 27.2% (without adding CaF 2 The hourly output is 5.5g) Then the morphology of the prepared product was observed by field emission scanning electron microscope, and the result showed that the prepared product was SnO 2 Nanowire, the product has high purity.
Embodiment 3
[0036] The mass fractions were 44% of Al+CuO powder (strictly according to the reaction ratio, that is, the molar ratio of 2:3), 52% of Sn powder and 4% of CaF 2 Mix the powder and stir evenly, then pour the powder into the powder chamber. Then, put it under the hydraulic testing machine, the preset pressure is 30MPa. Under the action of the upper and lower pressure heads, the powder is compacted. After reaching the preset pressure, the pressure is kept for 5 seconds, and then unloaded. Unscrew the bolts around the mold, take out the compacted powder and put it in figure 1 Inside the graphite crucible shown. Then sprinkle the ignition powder on the surface of the powder, insert the fuse to ignite the reaction. After the reaction is completed, the product is completely collected in a pre-placed collection device.
[0037] The collected product was weighed, and the yield was 8.4 g, a 20% increase in weight compared to the previous unpressurized (7 g unpressurized yield). Su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com