Goat inactivated vaccine and preparation method thereof
A technology for inactivated vaccines and goat pox, applied in biochemical equipment and methods, methods based on microorganisms, pharmaceutical formulations, etc., can solve problems such as long production cycle, low virus titer content of virus liquid, and complicated cell culture process, etc. To achieve the effect of avoiding seasonal restrictions, avoiding potential risks, and good immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 goat pox inactivated vaccine
[0024] 1. Preparation of spinner bottle cell virus:
[0025] Take a single layer of well-grown BHK-21 cells, discard the nutrient solution, digest with trypsin, add milk liquid containing 2% calf serum, and inoculate the goat pox virus seed used for production on the BHK-21 digested with trypsin. 21 In the cell suspension, make up the cell maintenance solution, culture at 37°C, and observe the cell changes every day. Viral cultures were harvested after approximately 20 hours when cytopathy reached 75%.
[0026] 2. Suspension cell virus preparation:
[0027] Take 1 branch of working seed cells, resuscitate and adhere to the wall for 2 passages, grow to a monolayer and then culture in suspension for 3 passages in shake flasks, when the cell concentration reaches 2×10 6 / ml or more were transferred to 5L or 100L bioreactors for cell expansion and culture until the cell concentration reached 2×10 6 / ml or mo...
Embodiment 2
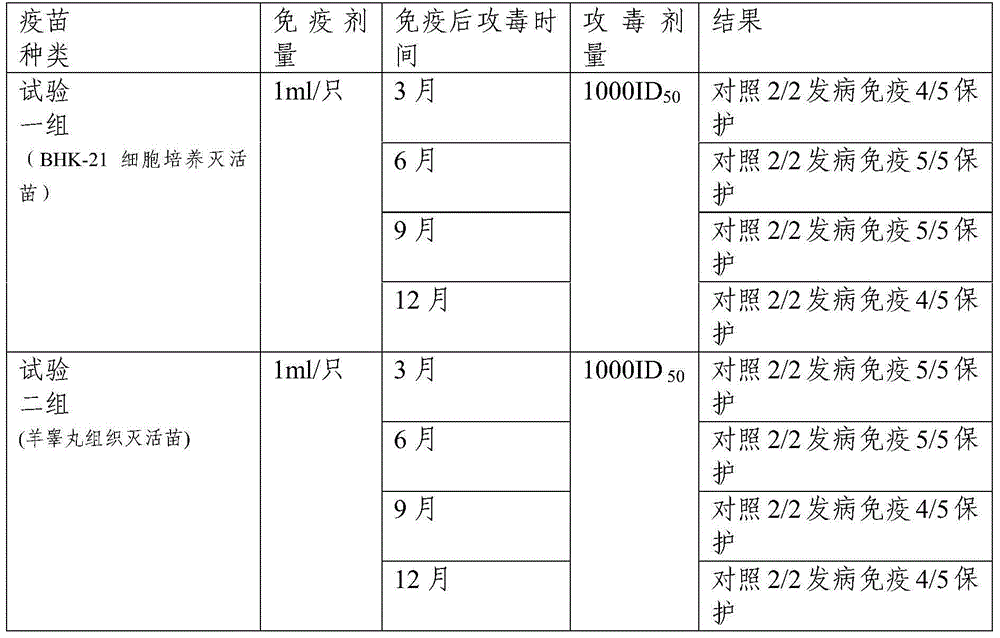
[0034] Example 2 Efficacy Test of Goat Pox Inactivated Vaccine
[0035] Screening of goat pox antibody-negative goats: through trial and error, a goat pox cell neutralization test was established: the goat serum to be tested was diluted 1:4 and mixed with 200 TCID 50 The goat pox venom was neutralized at 37°C for 1 hour, and inserted into well-growing bovine testicular cells. At the same time, a virus control, a negative control, and a goat pox positive serum control were set up to observe cell lesions. The virus control showed typical lesions, and the negative control and goat pox positive The serum control had no lesions, and the test was established. If lesions appear in the neutralized sample of the goat serum to be tested, the serum goat pox antibody is negative. Goat pox antibody was tested on the goats used in the experiment, and goats negative for goat pox antibody were successfully screened out.
[0036] According to Announcement No. 442 of the Ministry of Agricultu...
Embodiment 3
[0037] Example 3 Safety Test of Goat Pox Inactivated Vaccine
[0038] According to Ministry of Agriculture Announcement No. 442 "Veterinary Biological Products Registration Classification and Registration Data Requirements", the safety inspection of the 3 batches of vaccines prepared in Example 1 was carried out as follows:
[0039] Each batch of goat pox antibody-negative goats was vaccinated with 2 goats, 3ml / goat, injected subcutaneously in the ventral part, and the sheep were vaccinated, causing slight redness and swelling locally, which subsided after 3-5 days, observed for 15 days, and no secondary pox swelling appeared .
[0040] Healthy guinea pigs with a body weight of 350-450 g were subcutaneously injected with 2 ml each and healthy mice with a body weight of 18-22 g were each subcutaneously injected with 0.5 ml, and observed for 7 days.
[0041] Each batch of inactivated antigen injection goat pox antibody negative 3 goats, ventral intradermal injection, 1ml / goat, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com