A-π-a-type conjugated semiconducting polymer based on pyridazinopyrrole diketone and preparation method thereof
A pyridazinopyrrole diketo, conjugated polymer technology, applied in the field of A-π-A type conjugated polymer semiconductor materials, can solve the problem of no organic field effect transistors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of each monomer is described as follows:
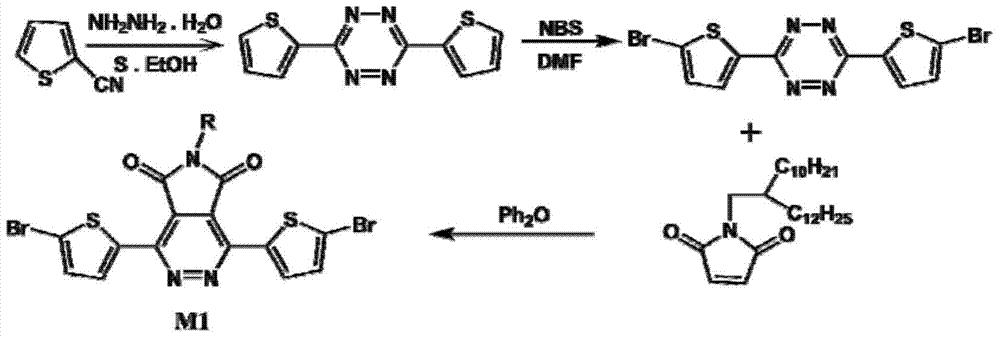
[0028] Preparation of conjugated dithiophene-pyridazinopyrrole diketopic bromide monomers
[0029] The schematic diagram of the synthesis route of conjugated bisthiophene-pyridazinopyrrole diketopic bromide monomer is as follows: figure 2 As shown, the dibromomonomer is prepared by the literature method, and the detailed preparation method can be found in the literature report (Organic Letters, 2014, 16, 6386.).
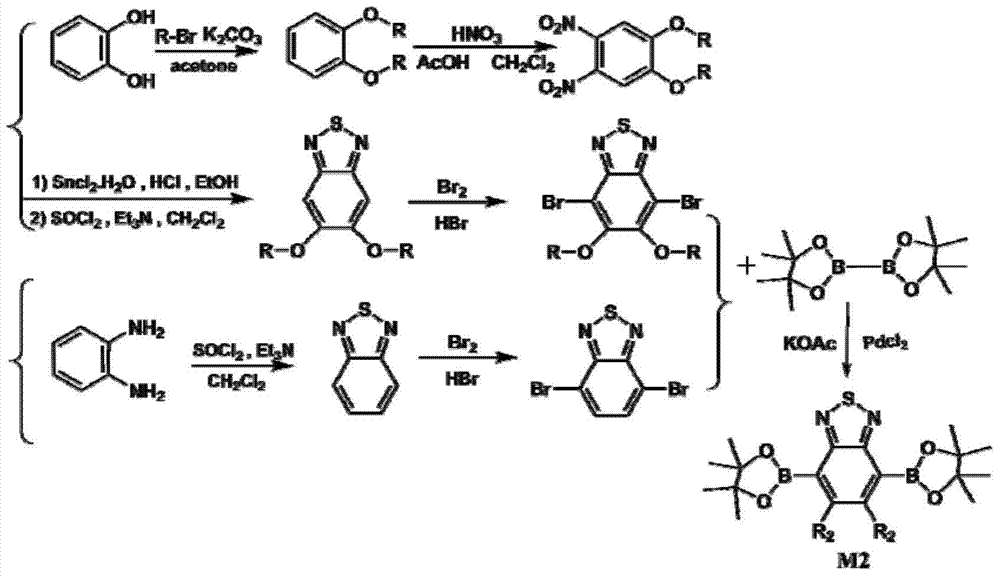
[0030] Preparation of benzothiadiazole diboronic acid di(pinacol) ester monomer
[0031] The synthetic route of benzothiadiazole diboronic acid bis (pinacol) ester monomer is as follows image 3 shown. For the detailed preparation method, refer to the literature report (European Journal Organic Chemistry, 2006, 21, 4924.).
Embodiment 1
[0032] Embodiment 1, synthetic polymer P1
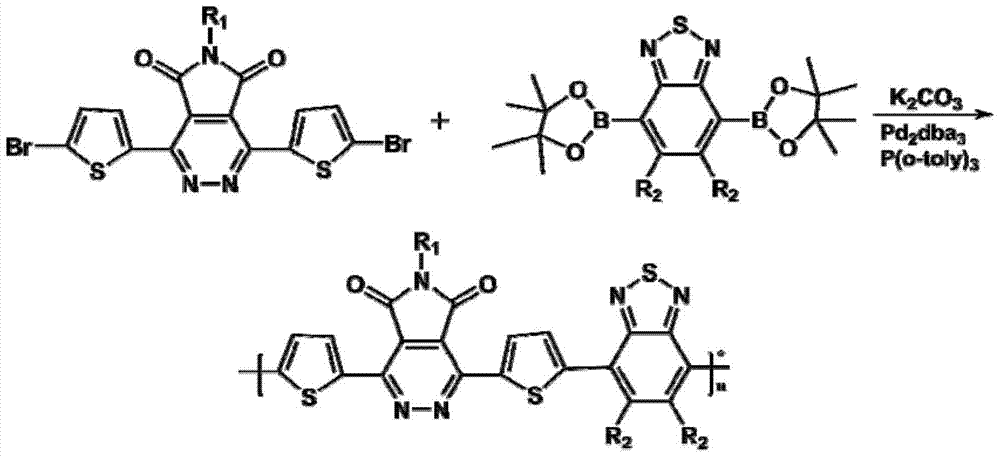
[0033] The synthetic route of polymer P1 is as follows Figure 4 As shown, the specific steps are: in a 100mL reaction flask, add 0.5mmol dibromine monomer and 0.5mmol diboronic acid bis(pinacol) ester monomer (R 1 and R 2 As shown in Table 1), then add 10mL anhydrous toluene, 5mL2mol / L K 2 CO 3 The solution was replaced with nitrogen for 40 minutes, and a 2% catalyst tris(dibenzylideneacetone)dipalladium was added, using tris(o-methylphenyl)phosphine as a ligand. React at 110°C for 48 hours, cool the reaction to room temperature, add 200mL of methanol to precipitate, filter the solid, use methanol and n-hexane Soxhlet extraction for 24 hours, and then use chloroform Soxhlet extraction for 24 hours, finally rotate the liquid, and precipitate with methanol to obtain polymer.
Embodiment 2-3
[0035] The specific steps are the same as in Example 1: in a 100mL reaction flask, add 0.5mmol dibromine monomer and 0.5mmol diboronic acid bis(pinacol) ester monomer (R1 and R2 are as shown in Table 1), add anhydrous toluene 10mL, 5mL 2mol / L K 2 CO 3Solution, replaced with nitrogen for 40 minutes, added 2% catalyst tris(dibenzylideneacetone) dipalladium, with tris(o-methylphenyl)phosphorus as ligand, reacted at 110°C for 48 hours, cooled to room temperature, added 200mL methanol Precipitation, the solid was extracted with methanol and n-hexane Soxhlet for 24 hours, and then extracted with chloroform for 24 hours. Finally, the liquid was rotary evaporated, and methanol precipitated to obtain polymers P2-P3. The specific structure is shown in Table 1.
[0036] Table 1
[0037]
[0038] In summary, the present invention relates to a semiconducting conjugated polymer based on bisthiophene-pyridazinopyrrole diketone and benzothiadiazole, and the structural feature of the nove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com