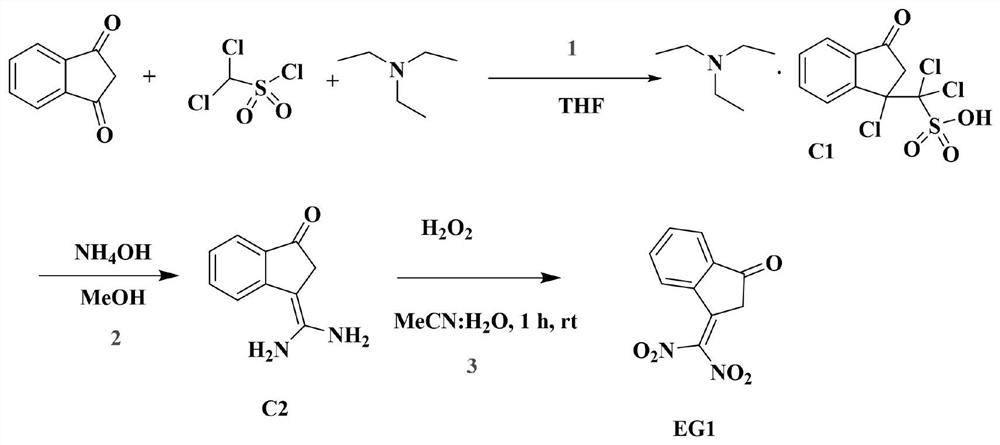
Compound with nitro-based electron withdrawing group, non-fullerene electron acceptor material and preparation method of non-fullerene electron acceptor material
A technology of electron acceptor materials and electron-withdrawing groups, which is applied in the field of organic solar cells, can solve problems such as unfavorable industrial production, operator injury, and easy pollution of the environment, so as to improve fill factor and short-circuit current, improve utilization rate, The effect of improving transmission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]A method for preparing a compound with a nitro-based electron-withdrawing group, comprising the steps of:
[0046] S1. Add 1H-indene-1,3(2H)-dione (10.0mmol) and dichloromethanesulfonyl chloride (11.0mmol) to tetrahydrofuran (100ml) solvent containing triethylamine (20.0mmol), at room temperature After stirring for 2 h, it was extracted with dichloromethane and the solvent was removed. The organic phase was dried with anhydrous sodium sulfate, and the obtained organic layer was purified by column chromatography to obtain intermediate product C1 (3.5 g, yield 64.0%), wherein ethyl acetate:methanol (3:1) was used as eluent. The intermediate product C1 is identified and analyzed by a mass spectrometer, and the result is: mass spectrum (EI): m / z C 16 h 22 C l3 NO 4 Theoretical value of S: 429.03; measured value: 430(M)+.
[0047] S2. Add C1 (10.0mmol) into a round bottom flask containing MeOH (30ml) and NH4OH (30.0mmol), stir for 6h, extract the obtained product with et...
Embodiment 2
[0051] A preparation method of a non-fullerene electron acceptor material, mainly formed by the Knoevenagel reaction of the compound EG1 and the compound T having an electron-withdrawing group based on a nitro group; the specific reaction steps are as follows:
[0052] S11. Add compound T and compound EG1 to a mixed solution of pyridine and chloroform for dissolution, then react at 65°C under nitrogen protection and reflux for 24 hours, cool to room temperature, introduce the mixture into methanol and filter;
[0053] S12. Purify the filtered residue by silica gel column chromatography, using dichloromethane:petroleum ether (1:1) as the eluent to obtain a solid product, that is, the non-fullerene electron acceptor material.
Embodiment 3
[0055] The electron-withdrawing group (EG1) prepared in Example 1 and the structural formula are The target compound T1, prepared by Knoevenagel reaction structural formula is The non-fullerene electron acceptor material T2.
[0056] Specific steps are as follows:
[0057] S11, compound T1 (0.15g, 0.15mmol), compound EG1 (0.20g, 0.85mmol), pyridine (1mL) and chloroform (45mL) were mixed and dissolved in a round bottom flask under nitrogen, and the mixed solution was heated at 65°C The reaction was stirred under the conditions for 24h. Then allowed to cool to room temperature, the mixture was introduced into methanol and filtered.
[0058] S12. Purify the residue by silica gel column chromatography, using dichloromethane:petroleum ether (1:1) as the eluent, and finally obtain the solid product non-fullerene electron acceptor material T2 (0.14g, yield 40%) .
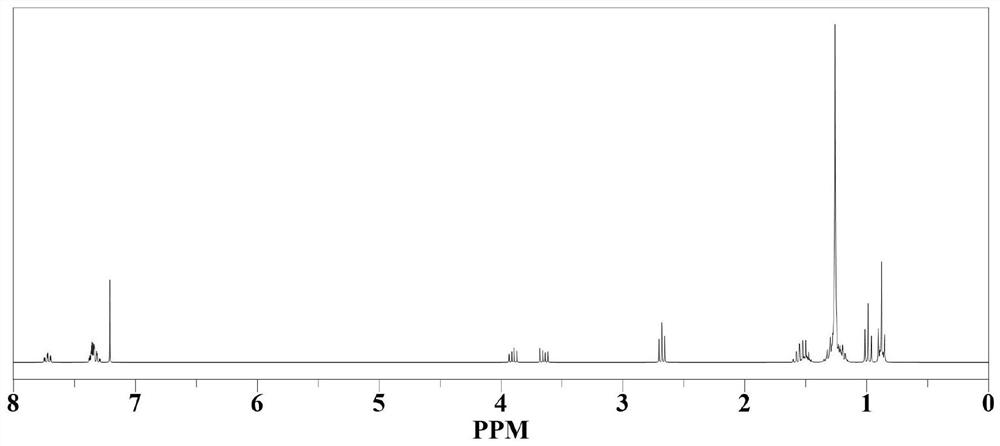
[0059] The non-fullerene electron acceptor material T2 is identified and analyzed by a mass spectrometer, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com