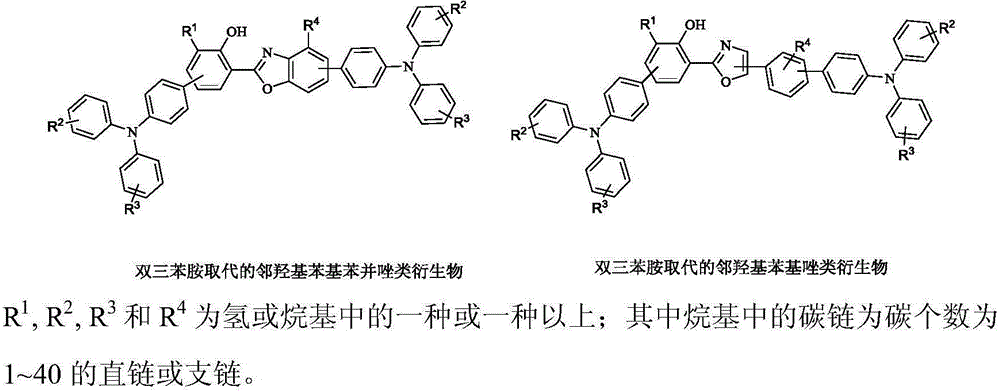
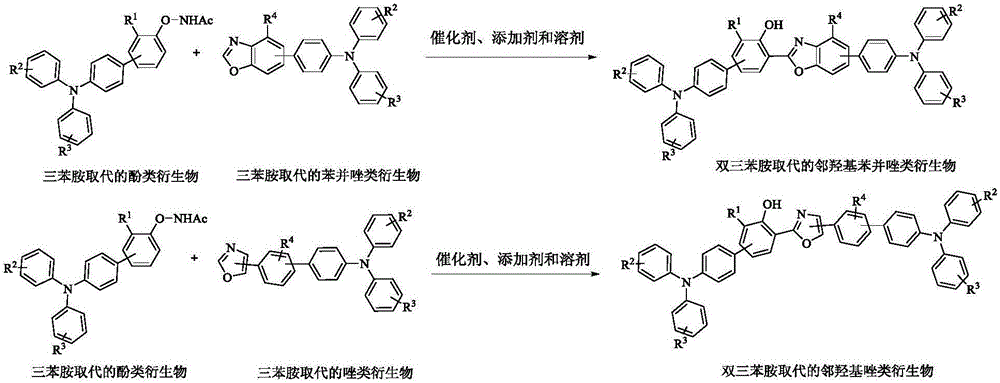
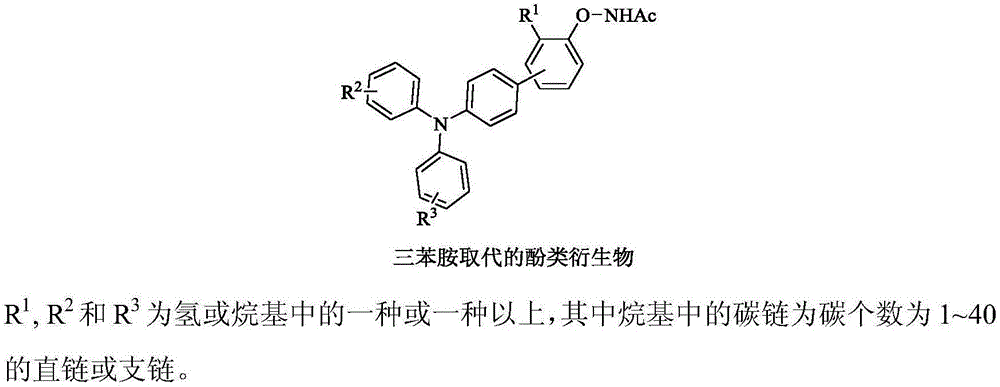
Preparation and application of bistriphenylamine substituted o-hydroxyphenylazole derivatives as organic monomolecular white light materials
A technology of o-hydroxyphenylazoles and molecular materials, which is applied in the field of organic single-molecule white light materials, can solve the problems of poor functional group tolerance, harsh reaction conditions, and difficulty in obtaining raw materials, and achieves reduction of operation difficulty, improvement of total yield, and high efficiency. quick results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of 4'-(diphenylamine)-3-(5-(4'-(diphenylamine)diphenyl-4-)oxazolyl-2-)diphenyl-4-phenol
[0038] (1) Will N -(4'-(Diphenylamine)diphenyl-4-oxo)acetimide (79 mg, 0.2 mmol), 4'-(oxazolyl-5)- N , N -Diphenyl-[1,1'-diphenyl]-4-amine (116 mg, 0.3 mmol), dichloro(pentamethylcyclopentadienyl)rhodium(III) dimer (5.0 mol %, 6.4 mg, 10 μmol), silver hexafluoroantimonate (20 mol%, 14.0 mg, 40 μmol), silver carbonate (0.4 equiv, 22 mg, 80 μmol), pivalic acid (2.0 equiv, 41 mg, 0.4 mmol), cesium pivalate (80 mol%, 38 mg, 0.16 mmol), N,N -Dimethylformamide (1 mL), stirred evenly under anhydrous and oxygen-free conditions, heated to 140 ° C, and reacted for 24 hours;
[0039] (2) After the reaction is completed, cool the reaction tube to room temperature, add ethyl acetate to dilute the reaction system, then filter through diatomaceous earth, and wash with ethyl acetate, combine the filtrates, wash with saturated saline, separate liquid, anhydrous sodium sulf...
Embodiment 2
[0040] Example 2: Synthesis of 4'-(diphenylamine)-3-(5-(4-(diphenylamine)-phenyl)benzoxazolyl-2)-5-methylbiphenyl-4-ol
[0041] (1) Will N -((4'-(diphenylamine)-3-methyl-[1,1'-diphenyl]-4)-oxy)acetimide (82 mg, 0.2mmol), 4'-(benzox Azolyl-5)- N , N -Diphenylaniline (109 mg, 0.3 mmol), dichloro(pentamethylcyclopentadienyl)rhodium(III) dimer (5.0 mol%, 6.4 mg, 10 μmol), silver hexafluoroantimonate (20 mol%, 14.0mg, 40 μmol), silver carbonate (0.4 equiv, 22 mg, 80 μmol), pivalic acid (2.0 equiv, 41 mg, 0.4 mmol), cesium pivalate (80 mol%, 38 mg , 0.16 mmol), N,N -Dimethylformamide (1 mL), stirred evenly under anhydrous and oxygen-free conditions, heated to 140 ° C, and reacted for 24 hours;
[0042] (2) After the reaction is completed, cool the reaction tube to room temperature, add ethyl acetate to dilute the reaction system, then filter through diatomaceous earth, and wash with ethyl acetate, combine the filtrates, wash with saturated saline, separate liquid, anhydrous sodi...
Embodiment 3
[0043] Example 3: The UV- Absorption Spectrum and Fluorescence Emission Spectrum
[0044] The compound 4'-(diphenylamine)-3-(5-(4'-(diphenylamine)diphenyl-4-)oxazolyl-2-)diphenyl-4-ol was dissolved in toluene and formulated into 1×10 -6 mol / L, put 2.5 mL into a cuvette, and measure the UV-absorption and fluorescence emission spectra. The absorption spectrum of compound 4'-(diphenylamine)-3-(5-(4'-(diphenylamine)diphenyl-4-)oxazolyl-2-)diphenyl-4-ol in toluene solution The maximum absorption peak is located at 309, 374 nm; the maximum emission peak of the fluorescence emission spectrum is located at 424, 539 nm, and its quantum efficiency is determined to be 60% (attached Figure 6 ). Compound 4'-(diphenylamine)-3-(5-(4'-(diphenylamine)diphenyl-4-)oxazolyl-2-)diphenyl-4-phenol and polystyrene (1 : 49) was dissolved in dichloromethane, spin-coated into a quartz plate to form a film, and measured UV-absorption and fluorescence emission spectra. Compound 4'-(diphenylamine)-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com