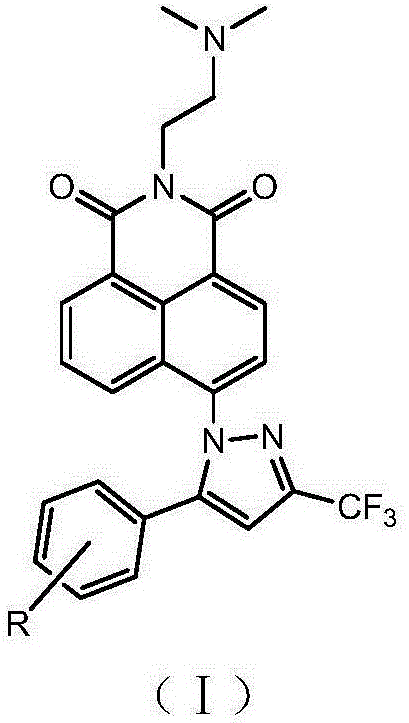
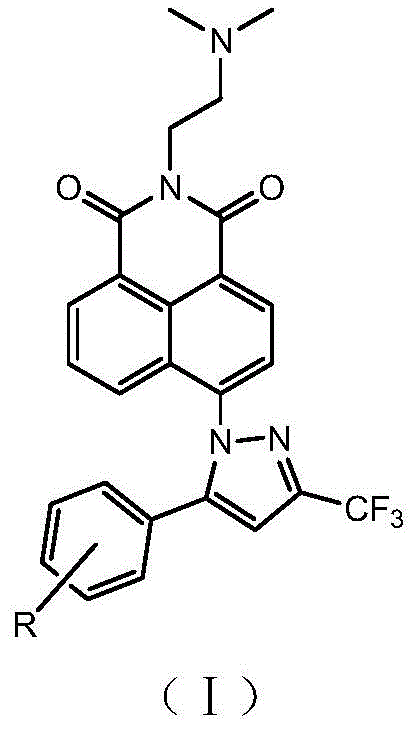
Naphthalimide-containing celecoxib derivatives with anti-tumor activity targeting DNA, pharmaceutical composition, preparation method and application thereof
A technology containing naphthalene diimide with anti-tumor activity, which is applied in the field of compounds and can solve problems such as weak activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
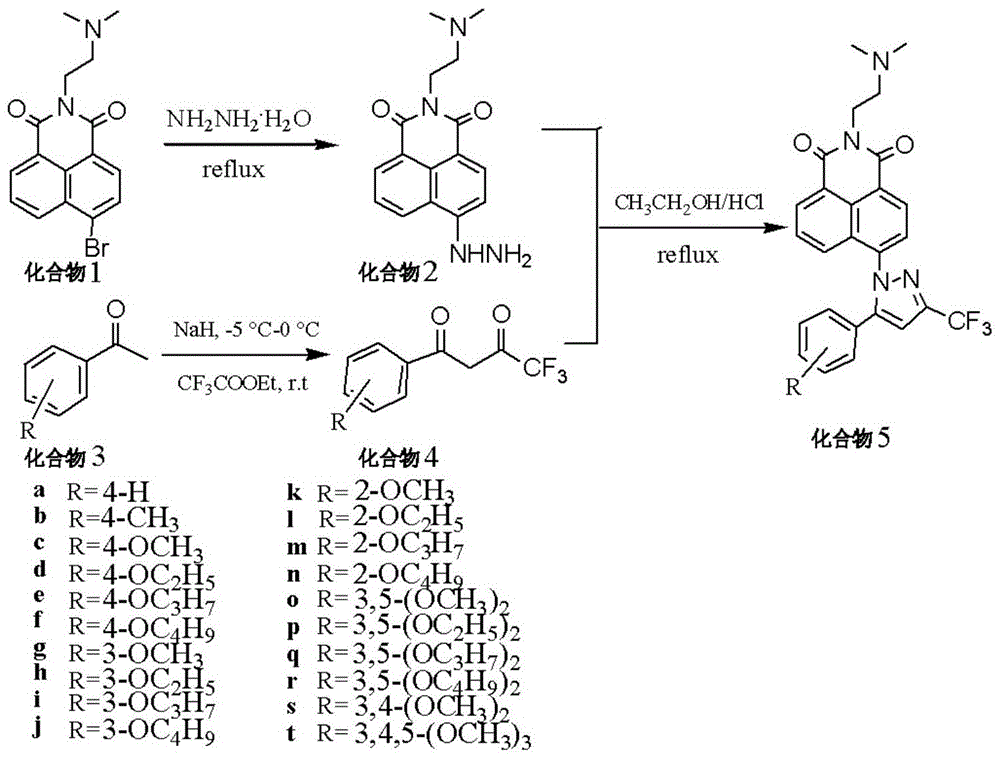
[0097] Preparation of compound 2:
[0098] Add compound 1 (N-(N,N-dimethylaminoethyl)-4-bromo-1,8-naphthalene diimide) 3g (0.88mmol) in a 100mL single-necked flask, volume percentage 80% Hydrazine hydrate aqueous solution 19mL, absolute ethanol 42mL, heated to reflux at 80°C until the reaction was identified by TLC as complete, cooled, and the solid was collected by suction filtration and dried in vacuo to obtain compound 2 (N-(N,N-dimethylaminoethyl)-4- hydrazino-1,8-naphthalimide), the yield was 92%.
Embodiment 2
[0100] Compound 5a [N-(N,N-dimethylaminoethyl)-4-(5-phenyl-3-trifluoromethyl-1H-pyrazole-1-yl)-1,8-naphthalimide 】Preparation:
[0101] ①Put 14.9mmol of acetophenone (compound 3a) and 30mL of dry THF into a 100mL round bottom flask, cool down to -5~0°C, add 0.715g (29.8mmol) of NaH in batches under the protection of nitrogen, at this temperature Stir for 30 min, add 3.175 g (22.4 mmol) of ethyl trifluoroacetate, stir at room temperature for 6 h, distill off the solvent under reduced pressure, add 30 mL of ice water for dilution, adjust the pH value of the solution to 6 with 1 mol / L HCl, and use ethyl acetate Extracted three times (10mL×3), recovered and combined the organic layers, then washed with 5mL of water, dried with anhydrous magnesium sulfate, precipitated, and dried the concentrated product to obtain compound 4a (4,4,4-trifluoro -1-phenyl-1,3-butanedione), yield 86%;
[0102] ②Add compound 4a (1mmol), compound 2 (1mmol), absolute ethanol 15mL, 1mol / L HCl 1.2mL in a ...
Embodiment 3
[0111] Compound 5b [N-(N,N-dimethylaminoethyl)-4-(5-(4-methylphenyl)-3-trifluoromethyl-1H-pyrazole-1-yl)-1, The preparation of 8-naphthalimide]:
[0112] ①Add 14.9mmol of 4-methylacetophenone (compound 3b) and 30mL of dry THF into a 100mL round bottom flask, cool down to -5~0°C, add 0.715g (29.8mmol) of NaH in batches under nitrogen protection, Stir at this temperature for 30 min, add 3.175 g (22.4 mmol) of ethyl trifluoroacetate, stir at room temperature for 6 h, evaporate the solvent under reduced pressure, add ice water to dilute the solution, and adjust the pH of the solution to 6 with 1 mol / L HCl. Extracted three times with ethyl acetate (10mL×3), recovered and combined the organic layers, washed the organic layer with 5mL of water, dried with anhydrous magnesium sulfate, desolventized, and dried the concentrated product to obtain compound 4b(4,4 , 4-trifluoro-1-(4-methylphenyl)-1,3-butanedione), the yield was 89%;
[0113] ②Add compound 4b (1mmol), compound 2 (1mmol), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com