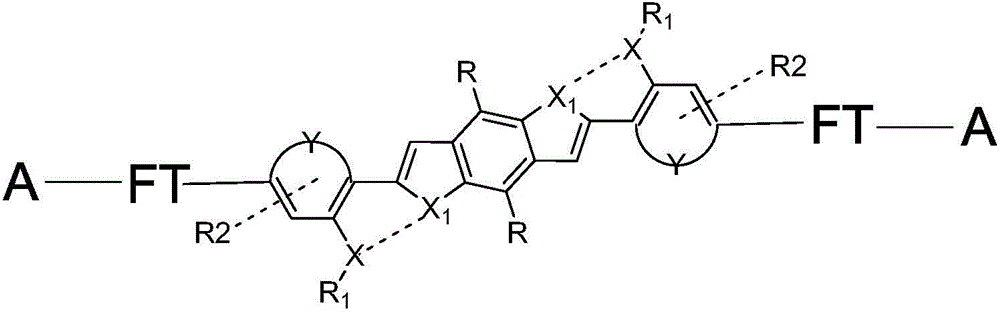
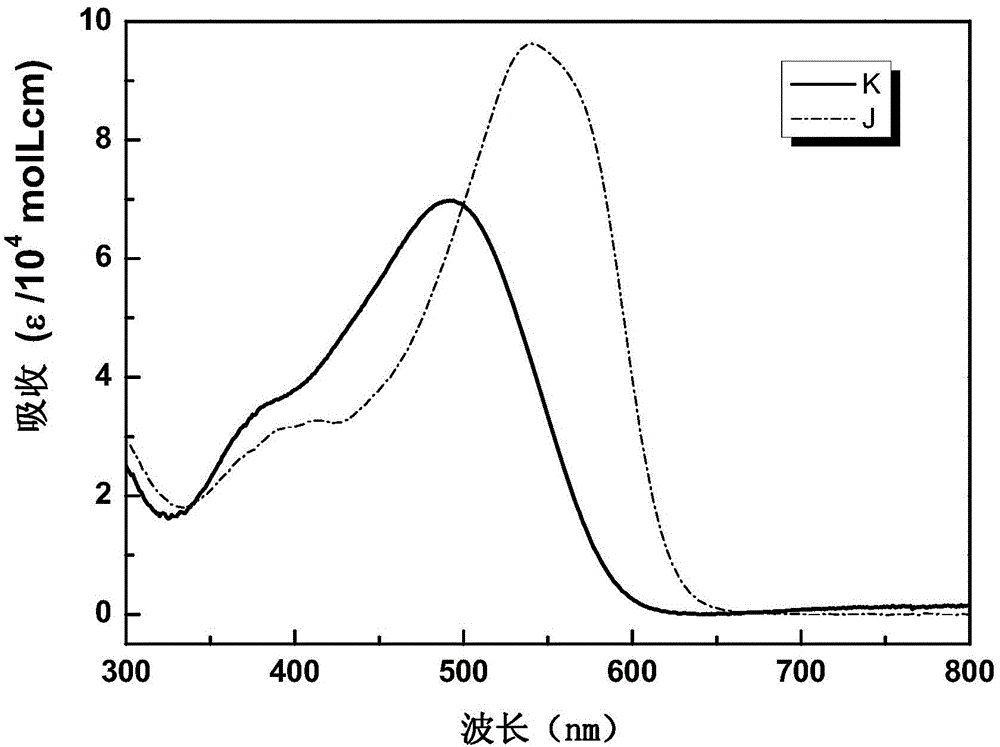
Organic small-molecular semiconductor material
A technology of small molecules and semiconductors, applied in the field of organic semiconductor materials, can solve the problems of reducing the spectral bandwidth of materials and reducing the effective conjugate length of molecules, and achieve the effects of long spectral absorption wavelength, extended effective conjugate length and good planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
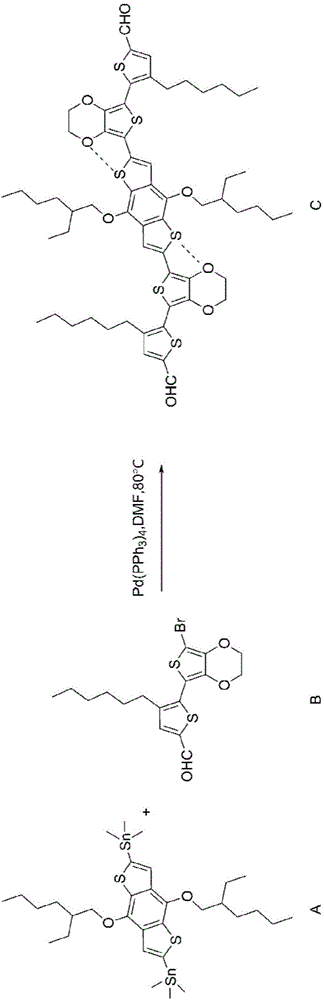
[0104] Embodiment 1 refers to image 3 , the synthesis and preparation process of the organic small molecule semiconductor material can be:
[0105] A (0.80g, 1.03mmol), tetrakis(triphenylphosphine) palladium (0.12g, 0.104mmol), B (0.95g, 2.28mmol), DMF (8mL), The reaction was carried out at 80°C for 20 hours. After the reaction was completed, the solid was collected by precipitation with methanol and purified by column chromatography to obtain the final product C (0.96g), with a yield of 83%. Wherein the characterization result of product is as follows:
[0106] MS (MALDI-TOF): Calcd, 1114.37; Found, 1114.58.
[0107] 1 H NMR (CDCl 3 ,400MHz):δ=9.85(s,2H),δ=7.64(s,2H),δ=7.58(s,2H),δ=4.46(s,8H),δ=4.19(d,4H),δ =2.86(t,4H),δ=1.87~1.79(m,2H),δ=1.75~1.68(m,8H),δ=1.56~1.43(m,16H)δ=1.36~1.30(m,8H) , δ=1.06(t,6H), δ=0.97(t,6H), δ=0.91(t,6H).
Embodiment 2
[0108] Embodiment 2: see Figure 4 , the synthesis and preparation process of the organic small molecule semiconductor material can be:
[0109] Add C (0.22g, 0.20mmol), 3-ethyl-2-thio-4-thiazolidinedione (71mg, 0.44mmol), 5 drops of piperidine, 10mL dry chloroform, reflux for 4h, water Interextract with chloroform, dry the organic phase, filter, spin dry the solvent, and recrystallize with ethanol to obtain the final product D (0.21g), with a yield of 74%. The characterization result of this product D is as follows:
[0110] MS (MALDI-TOF): Calcd, 1402.36; Found, 1402.62.
[0111] 1 H NMR (CDCl 3 ,400MHz):δ=8.00~7.70(s,2H),δ=7.70~7.40(s,2H),δ=7.40~7.10(s,2H),δ=4.60~4.30(s,8H),δ= 4.30~4.10(m,8H),δ=2.90~2.70(t,4H),δ=1.90~1.75(m,2H),δ=1.75~1.60(m,8H),δ=1.60~1.40(m, 16H) δ=1.40~1.20(m,8H), δ=1.10~0.90(m,24H).
Embodiment 3
[0112] Embodiment 3: see Figure 5 , the synthesis and preparation process of the organic small molecule semiconductor material can be:
[0113] Add A (0.11g, 0.14mmol), tetrakis(triphenylphosphine)palladium (17mg, 14.2μmol), E (0.24g, 0.31mmol), DMF (3mL) into a 25mL single-necked bottle under anhydrous and oxygen-free conditions, in The reaction was carried out at 80°C for 20 hours. After the reaction was completed, the solid was collected by precipitation with methanol and purified by column chromatography to obtain the final product F (0.21 g), with a yield of 84%.
[0114] The characterization result of this product F is as follows:
[0115] MS (MALDI-TOF): Calcd, 1804.80; Found, 1804.43.
[0116] 1 H NMR (CDCl 3 ,400MHz):δ=8.20(s,2H),δ=7.62(s,2H),δ=7.57(s,2H),δ=7.19(s,2H),δ=4.46(m,8H),δ =4.29(t,4H),δ=4.19(d,4H),δ=2.83(t,8H),δ=1.84~1.83(m,2H),δ=1.73~1.67(m,16H),δ= 1.44~1.25(m,56H), δ=1.06(t,6H), δ=0.98(t,6H), δ=0.89(t,18H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com