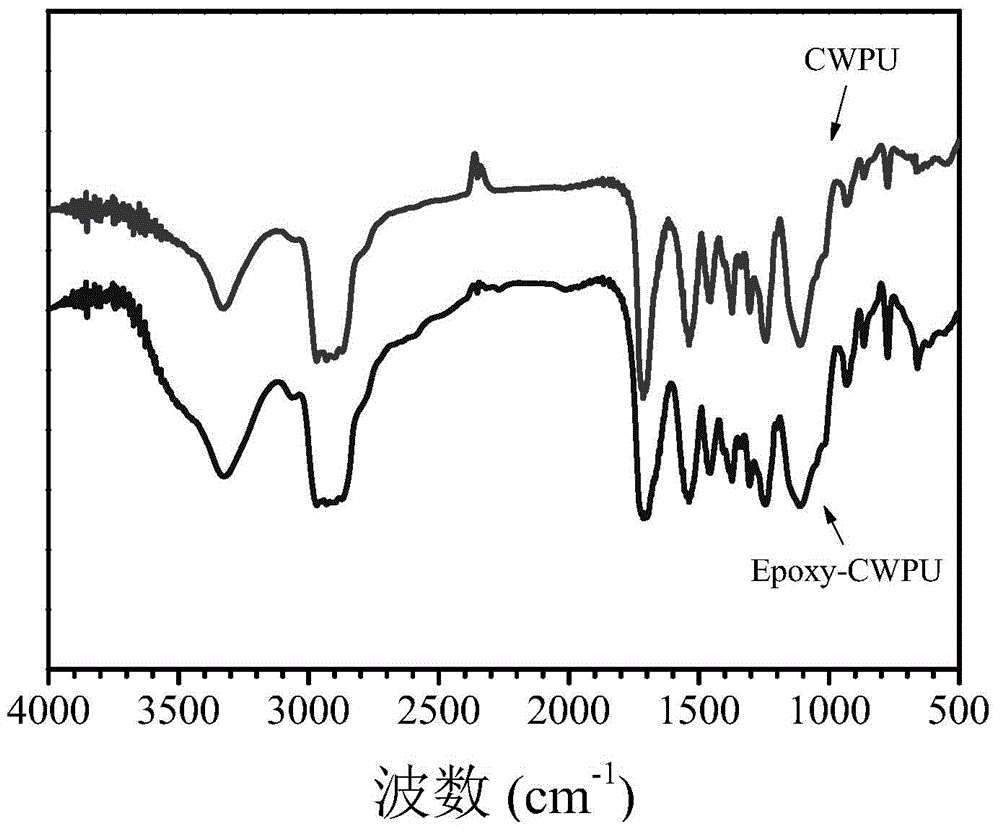
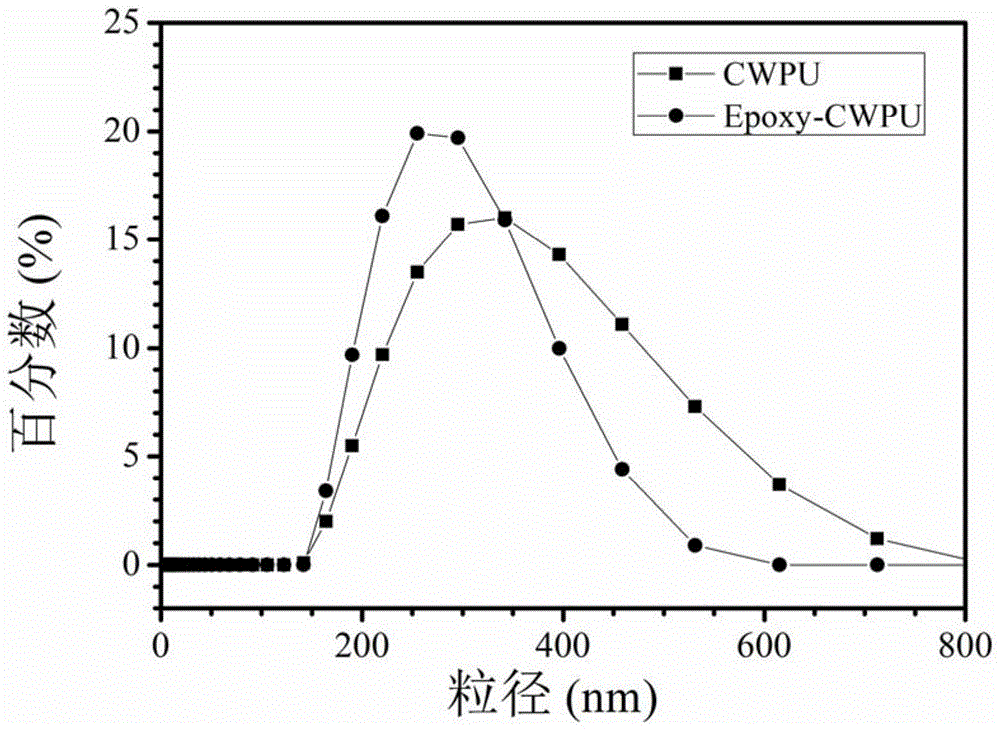
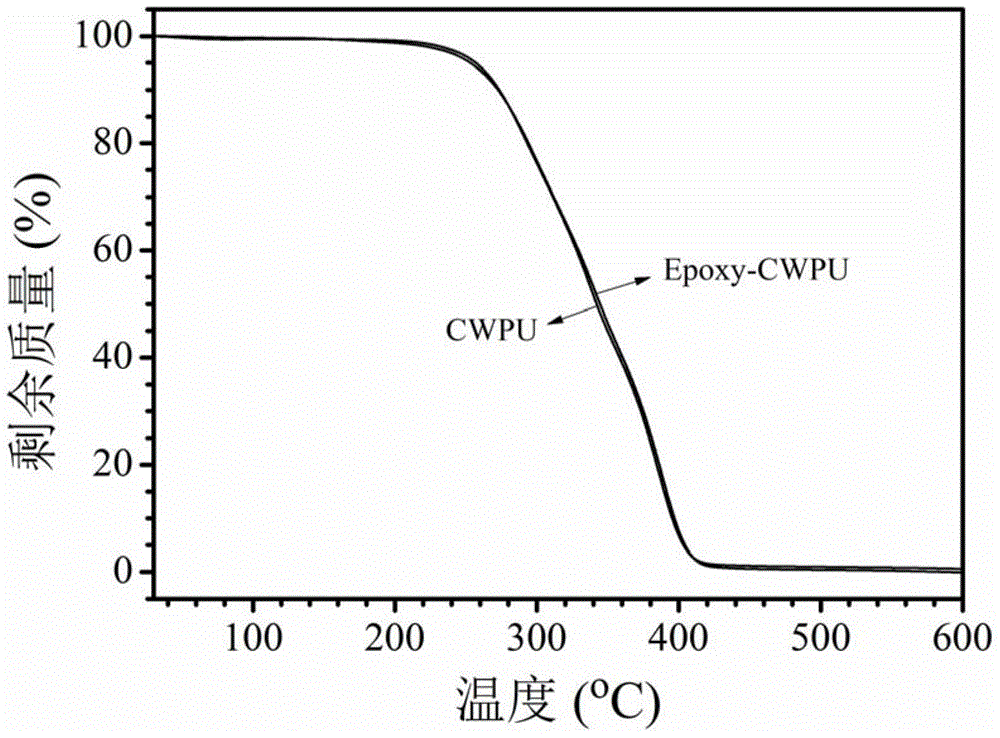
Preparation method of waterborne polyurethane based on glycidyl methacrylate
A technology of glycidyl ester and methacrylic acid, applied in the field of water-based polyurethane preparation, can solve the problems of difficult retention, unstable emulsion, and inability to further solidify, and achieve the effects of small particle size, narrow particle size distribution, and strong processing performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Dehydrate 47.2 grams of PPG (Mn=2000) at 100°C for 1.5 hours, then add 36.8 grams of IPDI, react at 90°C for 2 hours, add 4.8 grams of DEG, react at 70°C for 2 hours, and then add 11.0 grams at 50°C dropwise Add 0.045 g of BHT and 1.1 g of HEA to keep the reaction at 60°C for 1 hour to obtain a polyurethane with a double bond at the end group. Prepolymer; then add 0.05 g of AIBN and 3.0 g of glycidyl methacrylate to the polyurethane prepolymer containing double bonds at the end group, add 100 mL of methyl ethyl ketone, react at 60-75 ° C for 3 hours, cool down to 30 ° C, add After reacting 5.6 grams of acetic acid for 1-5 minutes, 242 mL of water was added under high-speed shearing, and after stirring for 5-30 minutes, the reaction product was desolventized butanone at 45 ° C and 0.01 MPa vacuum to obtain a solid content of 30 wt %. Block cationic water-based polyurethane emulsion Epoxy-CWPU with a glycidyl methacrylate content of 2.9 wt%.
[0028] In order to facilitat...
Embodiment 2
[0031] 50.0 g of PBA (M n =2000) dehydration at 100-120°C for 0.5-1.5 hours, then add 25.0 grams of TDI, add 2.7 grams of BDO after reacting at 80°C for 2 hours, react at 70°C for 2 hours, then at 50°C within 0.5-1 hour Add dropwise a mixed solution of 10.0 g MDEA and 40 mL methyl ethyl ketone, keep at 60 °C for 3 hours, add 0.035 g of HQ and 0.9 g of HEMA, and react at 60 °C for 1 hour to obtain a polyurethane prepolymer with double bonds at the end group; Add 0.03 g of AIBN and 5.1 g of glycidyl methacrylate to the polyurethane prepolymer containing double bonds, add 100 mL of butanone and react at 60-75 °C for 3 hours, then cool down to 30 °C, add 5.06 g of acetic acid for 5 minutes 219mL of water was added under stirring, and after stirring for 5-30 minutes, the reaction product was desolventized butanone under 45°C and 0.01MPa vacuum conditions to obtain a solid content of 30wt% and a glycidyl methacrylate content of 5.4wt%. Block cationic water-based polyurethane emulsi...
Embodiment 3
[0033] Dehydrate 50.0 grams of PTMG (Mn=2000) at 110°C for 0.5-1.5 hours, then add 25.3 grams of HDI, react at 90°C for 2 hours, add 3.3 grams of EG, react at 70°C for 2 hours, and then add 0.5 grams at 50°C Add a mixed solution of 8.0 g MDEA and 40 mL methyl ethyl ketone dropwise within 1 hour, keep the reaction at 65 °C for 3 hours, add 0.07 g BQ and 1.0 g HPA, and react at 60 °C for 1 hour to obtain a polyurethane prepolymer with double bonds in the terminal group; then Add 0.09 g of AIBN and 6.0 g of glycidyl methacrylate to the polyurethane prepolymer with double bonds in the terminal group, add 100 mL of methyl ethyl ketone and react at 70 ° C for 3 hours, then cool down to 30 ° C, add 4.03 g of acetic acid for 5 minutes and react Add 220mL of water under high-speed shearing, stir for 5-30 minutes, and remove the solvent butanone from the reaction product at 45°C and 0.01MPa vacuum to obtain a solid content of 30wt% and a glycidyl methacrylate content of 6.4 wt % block c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com