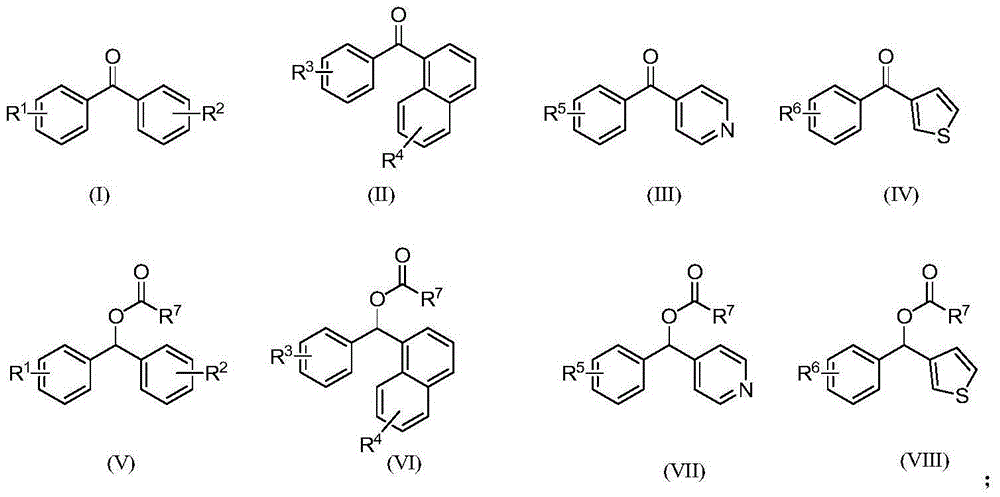
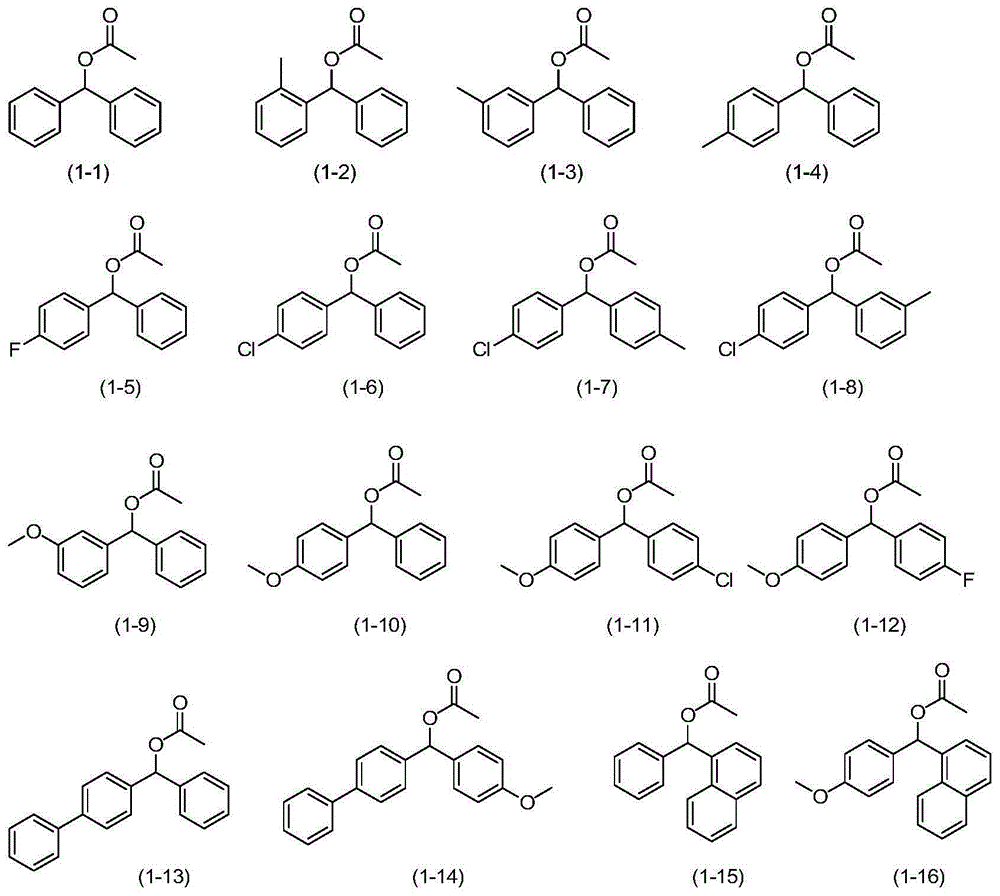
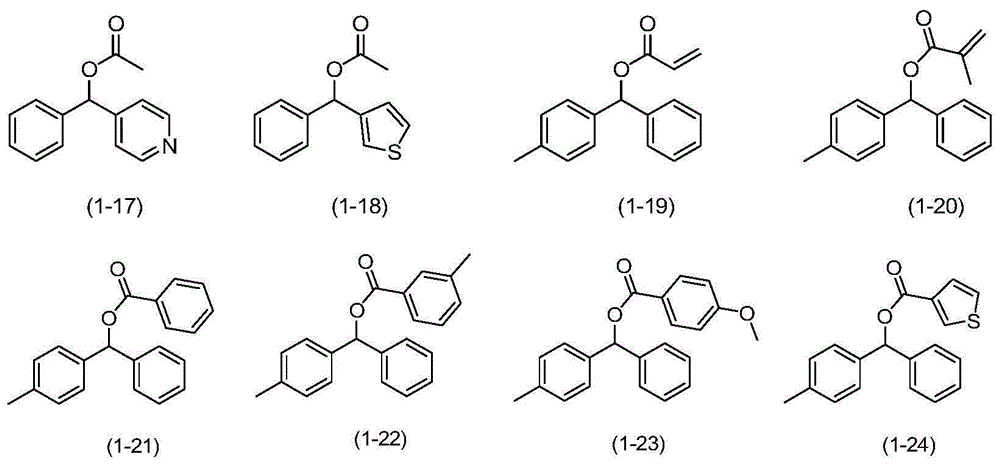
Catalytic oxidation synthesis method of diaryl ketone compound
A technology of diaryl ketone and synthesis method, which is applied in the field of catalytic oxidation synthesis of diaryl ketone compounds, can solve the problems of complicated operation, difficult separation, equipment corrosion, etc., and achieves simplified operation steps, convenient and safe operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of benzophenone (formula (2-1))
[0030] In a 200mL Teflon-lined autoclave, add benzhydryl acetate (formula (1-1), 0.45g, 2mmol), 1,1,2,2-tetrachloroethane (8ml), DDQ (0.14g, 0.6mmol), TBN (41.2mg, 0.4mmol), seal the autoclave, fill it with oxygen until the pressure gauge is 0.3MPa, put the autoclave into an oil bath preheated to 130°C, and react for 3h. After lowering the temperature and carefully releasing the pressure, the reaction solution was sampled and analyzed by gas chromatography (GC), the conversion rate was 100%, and the product selectivity was 99%. The reaction liquid was evaporated to remove the solvent under reduced pressure, passed through a silica gel column, and a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:200 was used as the eluent to collect the eluate containing the target compound, evaporated to remove the solvent, and then dried to obtain two Benzophenone 0.35g, the isolated yield of benzop...
Embodiment 2
[0031] Embodiment 2: the preparation of phenyl o-tolyl ketone (formula (2-2))
[0032] In a 200mL polytetrafluoroethylene-lined autoclave, add phenyl-o-tolylmethanol acetate (formula (1-2), 0.48g, 2mmol), 1,1,2,2-tetrachloroethane (8ml ), DDQ (0.14g, 0.6mmol), TBN (41.2mg, 0.4mmol), a closed autoclave, filled with oxygen until the pressure gauge is 0.3MPa, put the autoclave into an oil bath heated to 130°C in advance, and react 6h. After the temperature was lowered and the pressure was carefully released, the reaction solution was sampled and analyzed by gas chromatography (GC), the conversion rate was 97%, and the product selectivity was 99%. The reaction solution was evaporated to remove the solvent under reduced pressure, passed through a silica gel column, and the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:200 was used as the eluent to collect the eluate containing the target compound, evaporated to remove the solvent, and then dried to obtain b...
Embodiment 3
[0033] Embodiment 3: the preparation of phenyl m-tolyl ketone (formula (2-3))
[0034] In a 200mL polytetrafluoroethylene-lined autoclave, add phenyl-m-tolylmethanol acetate (formula (1-3), 0.48g, 2mmol), 1,1,2,2-tetrachloroethane (8ml ), DDQ (0.14g, 0.6mmol), TBN (41.2mg, 0.4mmol), a closed autoclave, filled with oxygen until the pressure gauge is 0.3MPa, put the autoclave into an oil bath heated to 130°C in advance, and react 3h. After lowering the temperature and carefully releasing the pressure, the reaction solution was sampled and analyzed by gas chromatography (GC), the conversion rate was 100%, and the product selectivity was 99%. The reaction solution was evaporated to remove the solvent under reduced pressure, passed through a silica gel column, and the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:200 was used as the eluent to collect the eluate containing the target compound, evaporated to remove the solvent, and then dried to obtain benzen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com