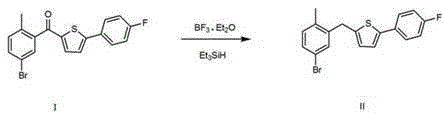
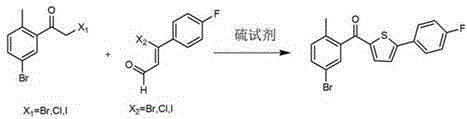
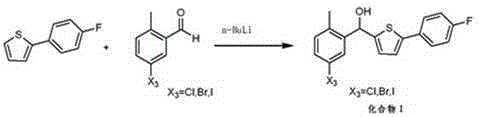Preparation method of canagliflozin intermediates
A technology for an intermediate, fluorophenylthiophene, is applied in the field of synthesis of pharmaceutical intermediates, and can solve the problems of difficulty in purchasing, low reaction yield and high cost, and achieve the effects of easy control of operating conditions, high product purity and stable reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: with Na 2 Add S (57.8g, 741mmol) into a 2L four-neck flask, then add 420ml of N,N-dimethylformamide to the system, and add N,N -Dimethylformamide (140ml) solution, dropwise, temperature controlled 65~75℃ and stirred for 1~2h, then slowly added dropwise 3-bromoacrolein (100g, 741mmol) in N,N-dimethylformamide (140ml) solution, the dropwise addition is completed and the temperature is controlled at 70-80°C to continue the reaction for 2-3 hours. After the HPLC detection is complete, the temperature is lowered to 0-10°C, and water (140ml) is dropped into the system. The system was filtered, the solid was recrystallized with methanol (420ml), cooled to 0-5°C for crystallization for 2 hours, filtered, and the solid was dried to obtain p-fluorophenylthiophene (122.8g, HPLC: 98.4%, yield 93%).
Embodiment 2
[0051] Embodiment 2: with Na 2 Add S (58.9g, 755mmol) into a 2L four-neck flask, then add 420ml of N,N-dimethylformamide to the system, and add N,N -Dimethylformamide (140ml) solution, dropwise, temperature controlled 65~75℃ and stirred for 1~2h, then slowly added dropwise 3-bromoacrolein (103g, 763mmol) in N,N-dimethylformamide (140ml) solution, the dropwise addition is completed and the temperature is controlled at 70-80°C to continue the reaction for 2-3 hours. After the HPLC detection is complete, the temperature is lowered to 0-10°C, and water (140ml) is dropped into the system. The system was filtered, the solid was recrystallized with methanol (420 ml), the temperature was lowered to 0-5°C for crystallization for 2 hours, and then filtered, and the solid was dried to obtain p-fluorophenylthiophene (125.4 g, HPLC: 98.9%, yield 95%) .
Embodiment 3
[0052] Embodiment 3: with Na 2Add S (60.7g, 778mmol) into a 2L four-neck flask, then add 420ml of N,N-dimethylformamide to the system, and add N,N -Dimethylformamide (140ml) solution, dropwise, temperature controlled 65~75℃ and stirred for 1~2h, then slowly added dropwise 3-bromoacrolein (110g, 815mmol) in N,N-dimethylformamide (140ml) solution, the dropwise addition is completed and the temperature is controlled at 70-80°C to continue the reaction for 2-3 hours. After the HPLC detection is complete, the temperature is lowered to 0-10°C, and water (140ml) is dropped into the system. The system was filtered, the solid was recrystallized with methanol (420 ml), the temperature was lowered to 0-5°C for crystallization for 2 hours, and then filtered, and the solid was dried to obtain p-fluorophenylthiophene (125.4 g, HPLC: 98.1%, yield 95%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com