Catalytic rate improving keratinase mutants and preparation method thereof
A technology of keratinase mutation and keratinase, applied in biochemical equipment and methods, botanical equipment and methods, hydrolytic enzymes, etc. Detergent and other problems, to achieve the effect of enhancing the anti-detergent SDS ability and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of recombinant bacteria expressing keratinase C-terminal shear mutants
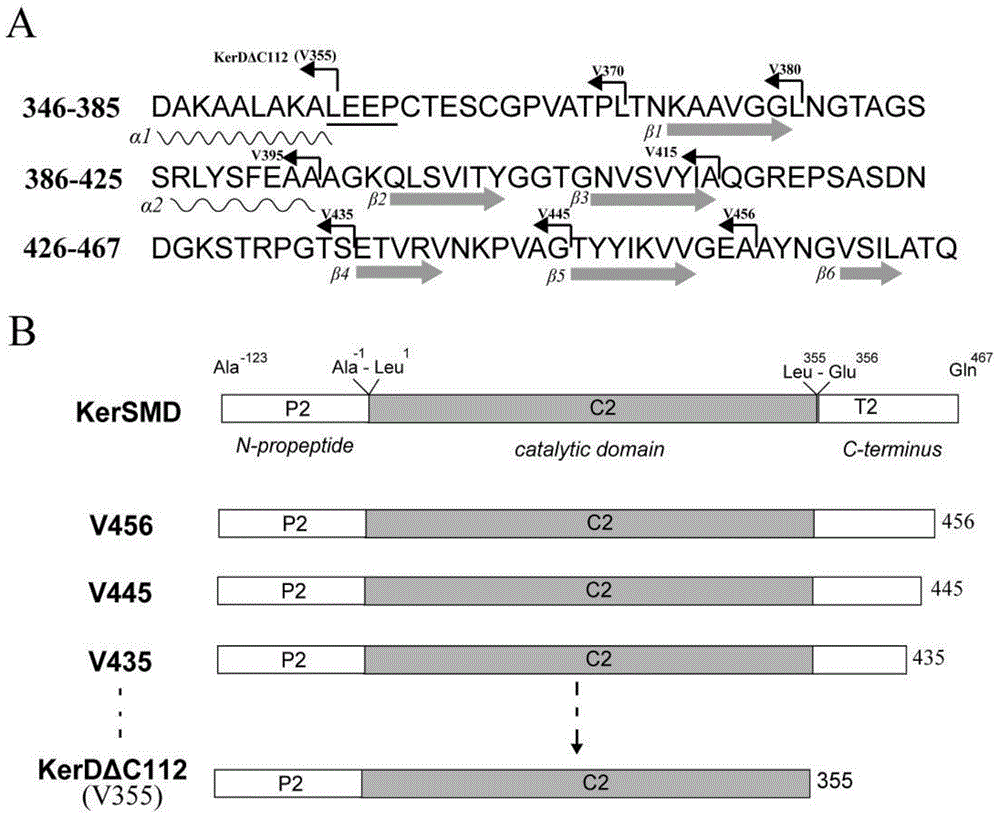
[0028] (1) According to the special β-sheet structure at the C-terminus, determine the cut site such as figure 1 . Then use a pair of primers (Table 1) carrying double restriction sites NcoI and XhoI, to contain Stenotrophomonas maltophilia (Stenotrophomonas maltophilia) BBE11-1 (on April 3, 2011 preserved in Chinese Type Culture The depository center, the deposit number is CCTCC No: M 2011193) The plasmid pET22b+kerSMD of the keratinase gene kerSMD was used as a template for PCR amplification. The reaction conditions are as follows: 95°C pre-denaturation for 5 minutes, followed by a cycle: 98°C denaturation for 10s, 55°C annealing for 10s, 72°C extension for 7min 50s, 30 cycles; 72°C extension for 1min 50s, and then cooling down to 12°C to obtain the final reaction solution . The DNA amplification enzyme used was Primer STAR from TaKaRa Company, and the formula was used ac...
Embodiment 2
[0034] Embodiment 2 recombinant bacterium fermentation produces keratinase mutant
[0035] Transform E.coli BL21 into E.coli BL21 with the recombinant expression vectors constructed according to the method in Example 1 to obtain the genetically engineered bacterium expressing keratinase; Medium 37°C liquid culture overnight, then insert LB fermentation liquid medium containing 100 μg / l ampicillin and culture at 37°C until OD 600 = 0.6, lower the temperature to 20°C for culture, add the inducer IPTG with a final concentration of 0.1 mM to induce culture, and centrifuge at 72 hours to obtain the supernatant enzyme solution, which is the crude enzyme solution.
Embodiment 3
[0036] Purification of embodiment 3 keratinase mutants and specific enzyme activity and catalytic rate determination
[0037](1) The recombinant Escherichia coli containing the plasmid of the mutant gene was induced and cultured at 20° C. for 3 days to obtain a crude enzyme solution.
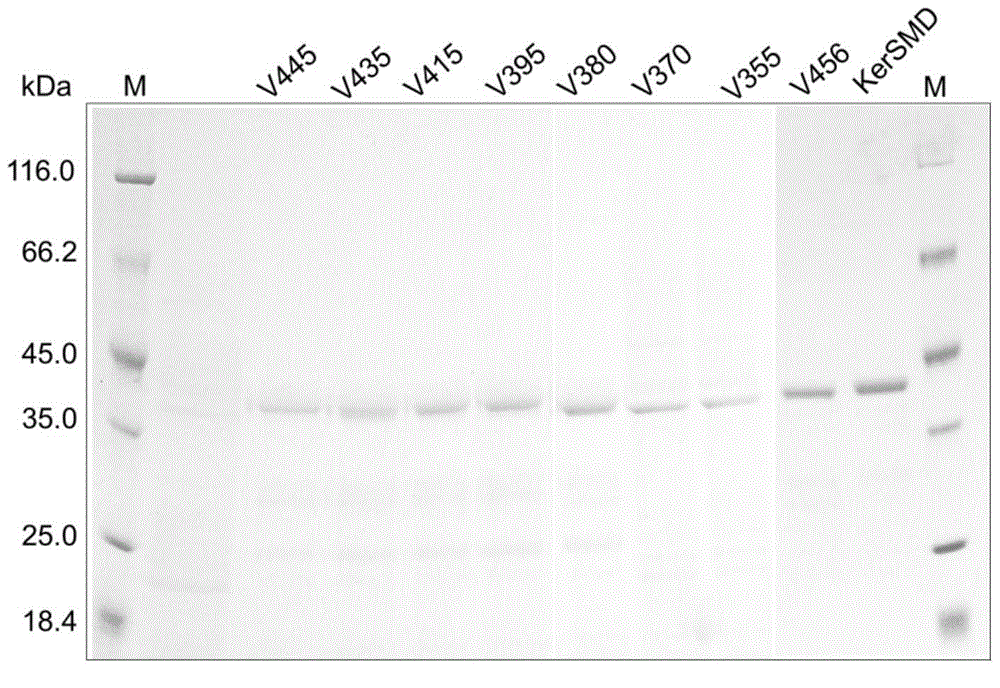
[0038] (2) Purify keratinase KerSMD with a purity of more than 90% and various mutants thereof from the crude enzyme solution by using an AKTA protein purifier (GE Company of the United States) and a nickel column of HisTrap FF crude 1 ml. SDS-PAGE of keratinase see figure 2 , the molecular weight of each protein is close to 43kDa.
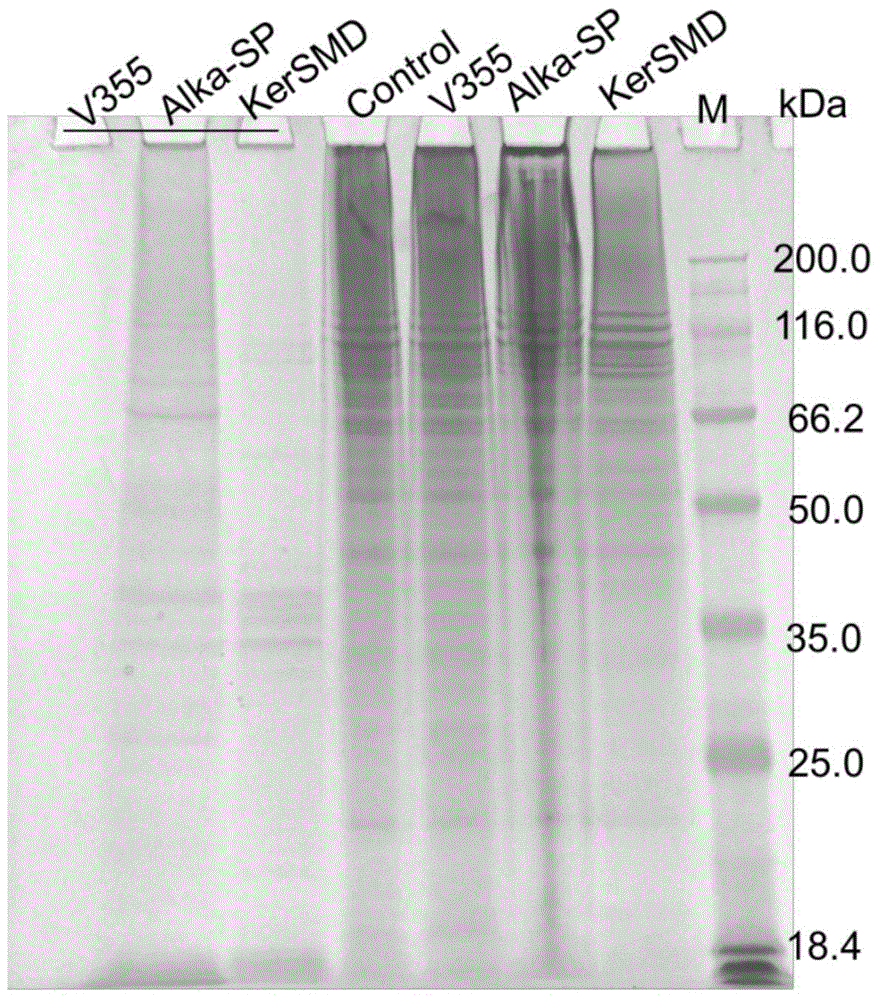
[0039] (3) The purified mutant keratinase was added to 50 mM Gly-NaOH buffer (pH 9.0) containing 1% (w / v) casein, incubated at 50° C. for different times, and the enzyme activity was determined. And the catalytic rate and other reaction kinetic parameters of the keratinase mutants to the synthetic substrate AAPF were determined.
[0040] (4) It can be seen from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com