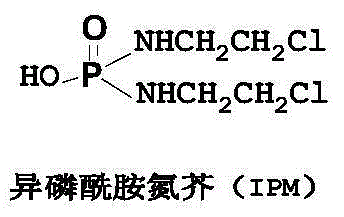
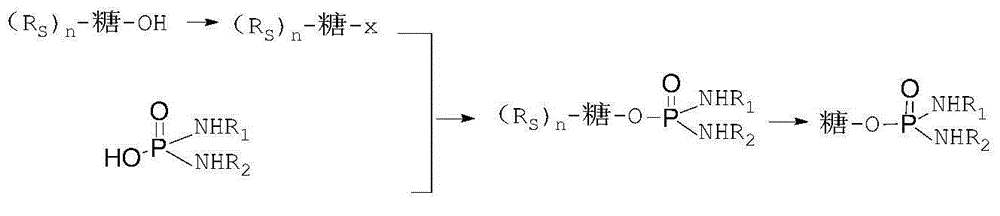
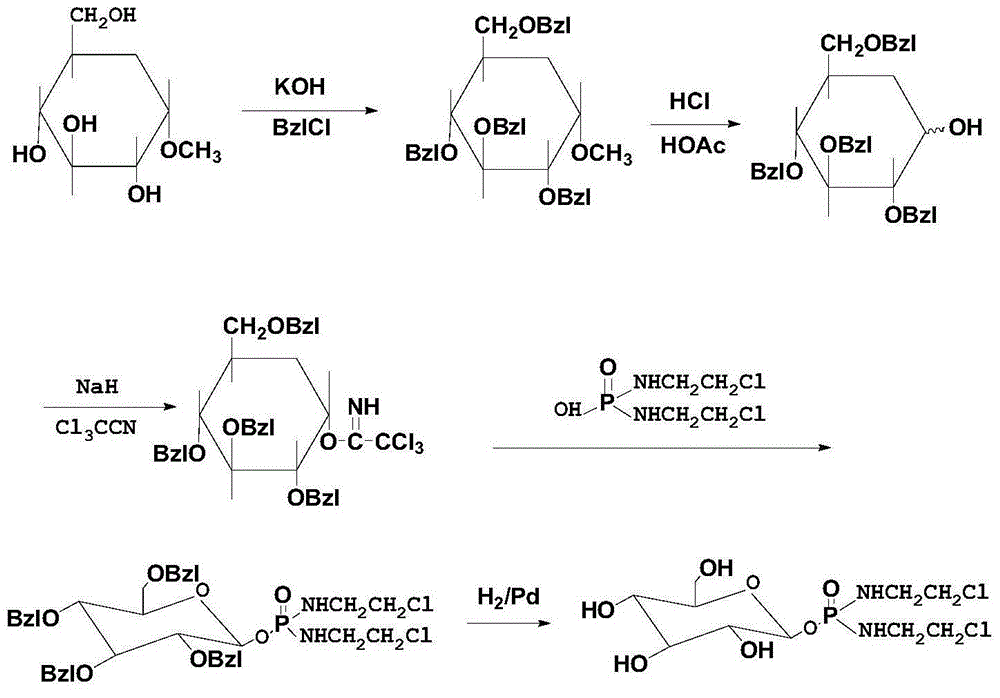
Method for preparing glufosfamide and its analogue by enzyme
A technology for glufosfamide and its analogues, which is applied in the field of compound biosynthesis, can solve problems such as the preparation methods of glufosfamide and its analogues that have not been seen, and achieve improved configuration selectivity, reduced generation of impurities, and increased reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This example relates to a method for preparing β-D-glufosfamide using enzymes.
[0040] Specific steps are as follows:
[0041](1) take by weighing the IPM of 10g and the β-D-glucose of 25g, adding concentration is that 0.3% sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution (pH6.0) makes the mass volume concentration of IPM be 20% (with g / ml meter);
[0042] (2) Add β-D-glucosidase equivalent to 0.05 times the amount of IPM to the solution obtained in step (1), and place it in a water bath at 20 to 30°C to start the reaction, and then take a sample every 4 hours, when β- When the D-glucose content is below 5%, stop the reaction, ultrafilter the enzyme component, concentrate the filtrate, and purify the product. After verification, the obtained product is indeed β-D-glufosfamide.
[0043] In this example, the yield of β-D-glufosfamide was 85%, and the purity was 99.2%.
Embodiment 2
[0045] This embodiment provides a synthetic method of β-D-mannose-IPM, specifically as follows:
[0046] (1) take by weighing the IPM of 10g and the β-D-mannose of 30g, adding concentration is that 0.4% sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution (pH6.5) makes the mass volume concentration of IPM be 25% ( in g / ml);
[0047] (2) Add β-D-mannosidase equivalent to 0.1 times the amount of IPM to the solution obtained in step (1), and place it in a water bath at 20-30°C to start the reaction, and then take samples every 4 hours, when the IPM content When it is less than 5%, the reaction is stopped, the enzyme component is ultrafiltered by an ultrafilter, the filtrate is concentrated, and the product is purified. After verification, the product obtained is indeed β-D-mannose-IPM.
[0048] In this example, the yield of β-D-mannose-IPM was 76%, and the purity was 99.4%.
Embodiment 3
[0050] This embodiment provides a synthetic method of β-D-galactose-IPM, specifically as follows:
[0051] (1) take by weighing the IPM of 10g and the β-D-galactose of 30g, adding concentration is that 0.4% sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution (pH7.5) makes the mass volume concentration of IPM be 40% ( in g / ml);
[0052] (2) Add β-D-galactosidase equivalent to 0.05 times the amount of IPM to the solution obtained in step (1), and place it in a water bath at 20-30°C to start the reaction, and then take samples every 4 hours, when β When the content of -D-galactose is less than 5%, the reaction is stopped, the enzyme component is ultrafiltered by an ultrafiltration machine, the filtrate is concentrated, and the product is purified. After verification, the obtained product is indeed β-D-galactose-IPM.
[0053] In this example, the yield of β-D-galactose-IPM was 80.3%, and the purity was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com