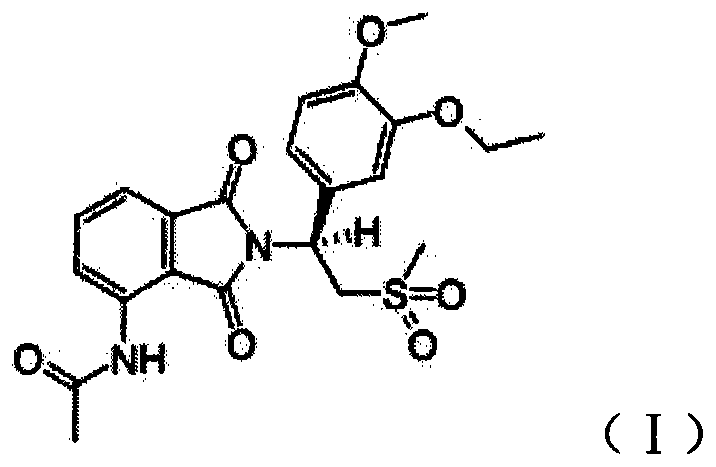
Tablets containing Apremilast active ingredients and vitro dissolution determination method thereof
A method of determination, technology of tablets, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Tablet prescription (1000 formulation units):
[0048]
[0049]
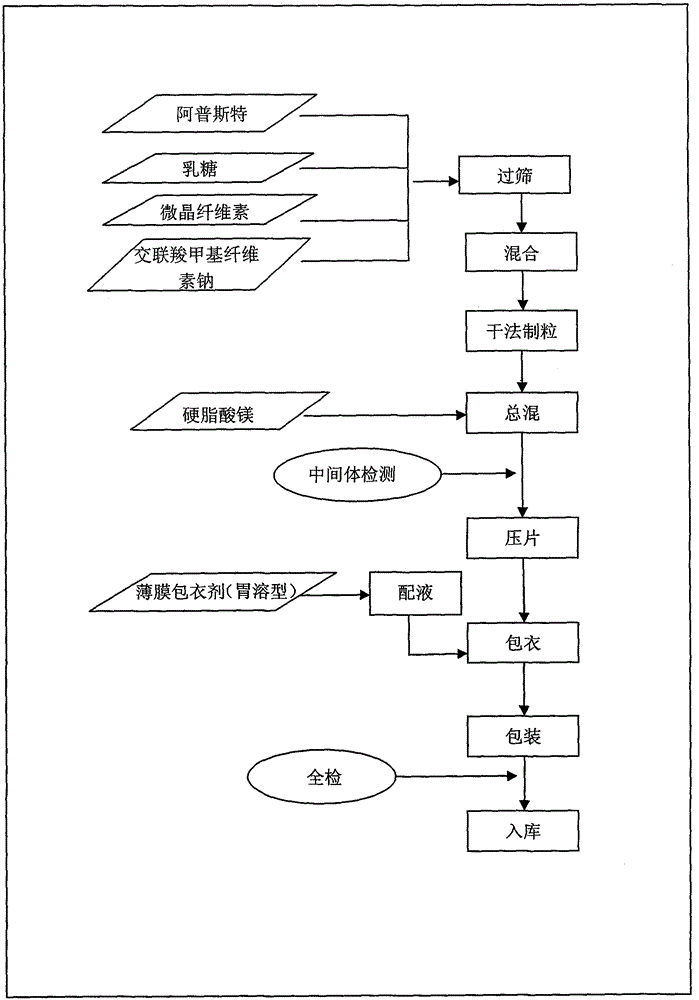
[0050] Preparation process (dry granulation and tabletting)
[0051] (1) Processing of raw and auxiliary materials: pass Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium through an 80-mesh sieve, and set aside.
[0052] (2) Mixing: Put Apremilast, lactose, microcrystalline cellulose and croscarmellose sodium in a high-speed mixing granulator, adjust the rotating speed to 150-200rpm, and mix for 15 minutes.
[0053] (3) Dry granulation: Put the mixed powder in a dry granulator, adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, and use a 20-30 mesh sieve for granulation , 20-30 mesh sieve for granulation.
[0054] (4) Total blending: Calculate the yield of granules after dry granulation, and then add converted lubricants and other additional auxiliary materials to the granules and mix evenly.
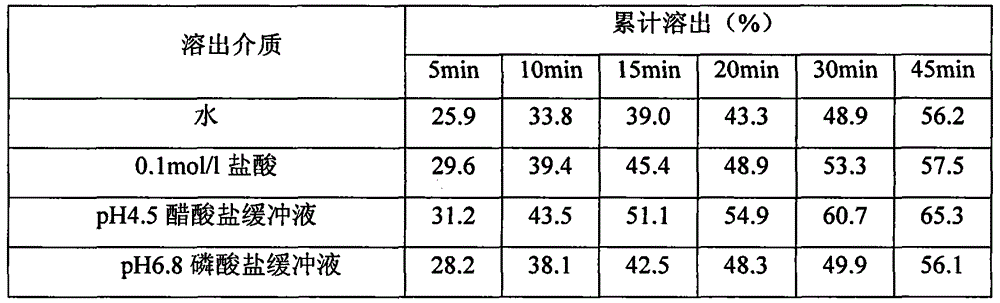
[0055] (5) Intermediate inspe...
Embodiment 2
[0059] Tablet prescription (1000 formulation units):
[0060]
[0061] Preparation process (dry granulation and tabletting)
[0062] (1) Processing of raw and auxiliary materials: pass Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium through an 80-mesh sieve, and set aside.
[0063] (2) Mixing: Put Apremilast, lactose, microcrystalline cellulose and croscarmellose sodium in a high-speed mixing granulator, adjust the rotating speed to 150-200rpm, and mix for 15 minutes.
[0064] (3) Dry granulation: Put the mixed powder in a dry granulator, adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, and use a 20-30 mesh sieve for granulation , 20-30 mesh sieve for granulation.
[0065] (4) Total blending: Calculate the yield of granules after dry granulation, and then add the converted lubricant and other external auxiliary materials to the granules and mix evenly.
[0066] (5) Intermediate inspection [co...
Embodiment 3
[0070] Tablet prescription (1000 formulation units):
[0071]
[0072] Preparation process (dry granulation and tabletting)
[0073] (1) Processing of raw and auxiliary materials: pass Apremilast, lactose, microcrystalline cellulose, and croscarmellose sodium through an 80-mesh sieve, and set aside.
[0074] (2) Mixing: Put Apremilast, lactose, microcrystalline cellulose and croscarmellose sodium in a high-speed mixing granulator, adjust the rotating speed to 150-200rpm, and mix for 15 minutes.
[0075] (3) Dry granulation: put the mixed powder in a dry granulator, adjust the roller pressure to 2.0-4.0MPa, the roller speed to 10-15rpm, the cutter speed to 10-15rpm, and use a 30-mesh sieve for granulation. Mesh sieve for whole grains.
[0076] (4) Total blending: Calculate the yield of granules after dry granulation, and then add converted lubricants and other additional auxiliary materials to the granules and mix evenly.
[0077] (5) Intermediate inspection [content, moi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com