Drug for treatment of type II diabetes
A technology for type 2 diabetes and drugs, applied in drug combinations, metabolic diseases, pharmaceutical formulations, etc., can solve the problems of unsatisfactory clinical efficacy of western medicine, carcinogenicity, and difficult for patients to adhere to.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
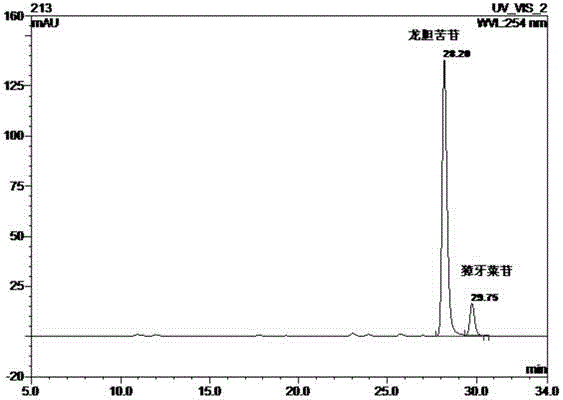
[0035] Example 1 Preparation process of Jinbuhuan extract and detection of extract components, drug efficacy experiment
[0036] 1. Extraction process:
[0037] Jinbuhuan 4.8kg was extracted and refluxed three times with 95% methanol aqueous solution, each time for 4 hours, filtered, combined extracts, and concentrated under reduced pressure to obtain 1227.68g of dark brown total extract.
[0038] Suspend the extract in water, extract with petroleum ether, dichloromethane, ethyl acetate, and n-butanol in sequence, and evaporate the four extracts to dryness to obtain extract, in which 74.21 g of petroleum ether extract, dichloromethane layer Extract 298.81g, ethyl acetate layer extract 236.15g, n-butanol layer extract 401.79g, water layer extract 193.29g.
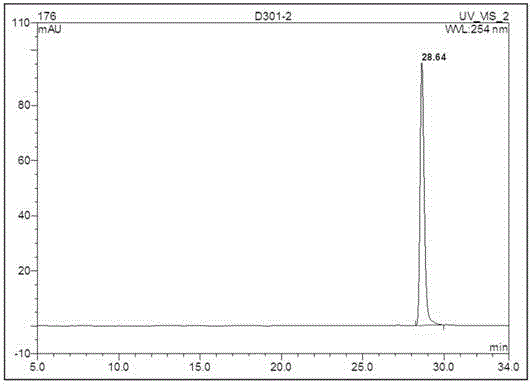
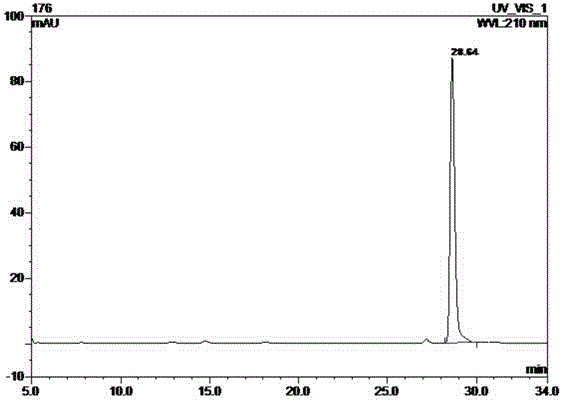
[0039] Take 50 g of the ethyl acetate layer extract and pass it through a normal-phase silica gel column, and use a gradient of dichloromethane-methanol (wherein the volume ratio of dichloromethane and methanol is 30:1, 9:1...
Embodiment 2
[0042] Example 2 In vitro anti-diabetic experiment of Jinbuhuan total extract
[0043] (1) HL1C rat liver cancer cells (hereinafter referred to as HL1C cells) are a kind of rat liver tumor H4IIE cell line, which contains a stably transfected Pck1 promoter gene, which can promote the expression of chloramphenicol acetyltransferase, and is suitable for use in Research on the Pck1 gene, the target of antidiabetic drugs. HL1C cells were cultured in high glucose DMEM containing 10% fetal bovine serum (4.5g / L glucose). Place it at 37°C with 5% CO 2 , Incubate in an incubator with saturated humidity, change the medium every 24 hours, and cultivate for 3 to 5 days for experimental use.
[0044] (2) After HL1C cells were co-incubated with different concentrations of total extracts, total RNA was extracted with Trizol, and then cDNA was extracted with a reverse transcription kit, and the level of Pck1 mRNA was detected by SYBR Green fluorescent probe method. The detection conditions w...
Embodiment 3
[0051] Example 3 Anti-type II diabetes experiment of gentiopicroside and / or swertiroside in vitro
[0052] (1) HL1C rat liver cancer cells were cultured in vitro in DMEM containing 10% fetal bovine serum. Place it at 37 °C with 5% CO 2 , incubating in an incubator with saturated humidity, changing the medium every 24 hours, and culturing for 3 to 5 days for experimental use.
[0053] (2) After HL1C cells were co-incubated with 30 μg / mL gentiopicroside for 6 hours, the total RNA was extracted by Trizol, and then the cDNA was extracted with a reverse transcription kit, and the Pck1 mRNA level was detected by the SYBR Green fluorescent probe method. °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
[0054] (3) HL1C cells were mixed with gentiopicroside, swertipicroside, and different concentrations of gentiopicroside and swertipicroside (the mass ratios of the two were 2:1, 5:1, 10:1, 1 :10, 1:5 and 1:2) mixtures were incubated for 15 min, then diges...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com