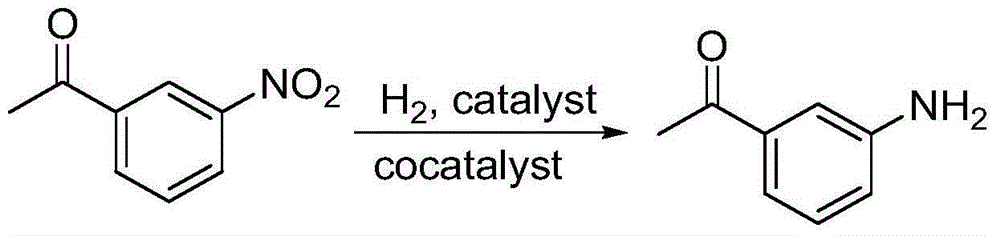
Synthetic method for 3-aminoacetophenone
A technology of aminoacetophenone and a synthesis method, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problems of polluted environment, high operation and maintenance cost, difficult purification and the like, and achieves the effects of high yield, low operation and maintenance cost, and simple technological process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of 3-aminoacetophenone, its step is as follows:
[0026] (1) Preparation of additives: Disperse and dissolve 20 g of cobalt nitrate into 100 ml of ethanol, then add 5 g of magnesium oxide nanopowder, stir and sonicate at 40 Hz for 30 minutes, evaporate the ethanol solvent to dryness, and vacuum dry at 100 ° C After 10 hours, it is finally ground into a fine powder with a particle size of 0.5-2 microns to obtain an auxiliary agent.
[0027] (2) Add 40g of 3-nitroacetophenone, 160g of methanol, 3.5g of 1% palladium carbon catalyst and 2g of the auxiliary agent prepared in step (1) to the autoclave successively, at 60-70°C, pressure 0.5-0.6 Hydrogenation reaction under MPa, heat preservation reaction for 45 minutes, cooling to room temperature, filtration, and precipitation of the filtrate to obtain 3-aminoacetophenone with a yield of 97%.
Embodiment 2
[0029] A kind of synthetic method of 3-aminoacetophenone, its step is as follows:
[0030] (1) Preparation of additives: Disperse and dissolve 20g of nickel nitrate in 100ml of ethanol, then add 5g of magnesium oxide nanopowder, stir and sonicate at 40 Hz for 30 minutes, evaporate the ethanol solvent to dryness, and vacuum dry at 100°C After 10 hours, it is finally ground into a fine powder with a particle size of 0.5-2 microns to obtain an auxiliary agent.
[0031] (2) Add 40g of 3-nitroacetophenone, 160g of methanol, 3.5g of 1% palladium carbon catalyst and 2g of the auxiliary agent prepared in step (1) in the autoclave, at 60-70°C, pressure 0.5-0.6 Hydrogenation reaction under MPa, heat preservation reaction for 45 minutes, cooling to room temperature, filtration, and precipitation of the filtrate to obtain 3-aminoacetophenone with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com