Method for chiral spirophosphonate catalyzed synthesis of optically active 2H-1,4-benzoxazine-2-one derivative
A technology of spirophosphoric acid catalysis and spirophosphoric acid catalyst, which is applied in directions such as organic chemistry to achieve the effects of convenient operation, mild reaction conditions and reduced preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
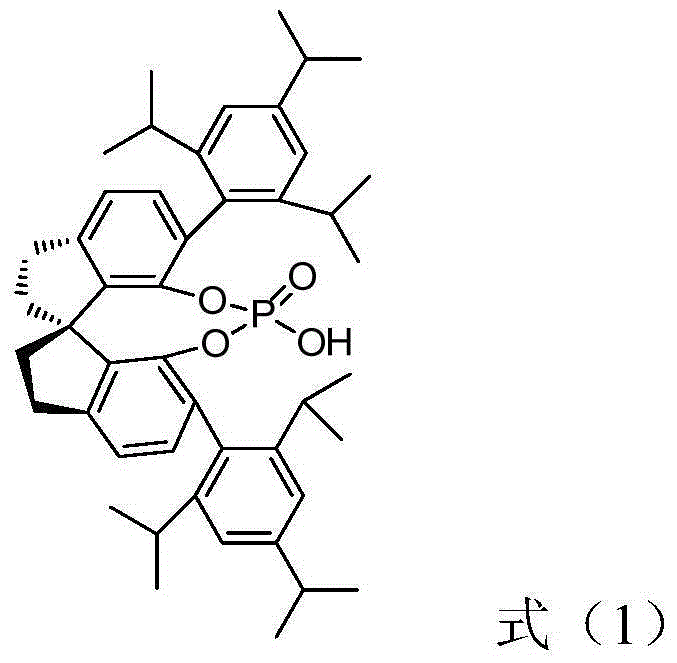
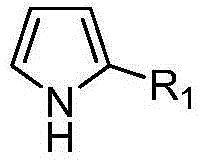
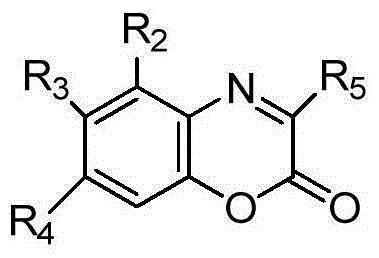
[0038] Add 3-trifluoromethyl-benzoxazinone (0.05mmol), pyrrole (0.06mmol), (R)-spirocyclic phosphoric acid (0.0025mmol) shown in structural formula (1) (cas: 1372719- 95-3), inject 0.5mL toluene, and react at room temperature for 12 hours. After the reaction is completed, directly use silica gel column chromatography, and the eluent is ethyl acetate / petroleum ether=1:8 to obtain the corresponding optically active 2H-1 , 4-benzoxazin-2-one derivatives, yield 93%; Product characterization is as follows:
[0039] Structural formula:
[0040]
[0041] Properties: yellow solid;
[0042] m.p.104.1-105.2°C;
[0043] Optical purity: 93%ee;
[0044] HPLC analysis conditions: (Dailu chiral column, the same below) Chiralpak OD-H (n-hexane / i-PrOH=90 / 10, 0.8mL / min), t R (minor)10.978min,t R (major) 16.172min;
[0045] Optical rotation: [α] D 20 =-190.1 (c=0.4, CH 2 Cl 2 );
[0046] 1 H NMR (400MHz, CDCl 3 )δ=8.77(s,1H),7.05–6.97(m,1H),6.95–6.84(m,2H),6.84–6.77(m,2H),6.26–6....
Embodiment 2
[0049] Add 6-fluoro-3-trifluoromethyl-benzoxazinone (0.05mmol), pyrrole (0.06mmol), (R)-spirocyclic phosphoric acid (0.0025mmol) shown in structural formula (1) in the reaction flask, Inject 0.5mL of benzene and react at room temperature for 24 hours. After the reaction is completed, directly use silica gel column chromatography with ethyl acetate / petroleum ether=1:8 as the eluent to obtain the corresponding optically active 2H-1,4-benzo Oxazin-2-one derivatives, yield 95%; product characterization as follows:
[0050] Structural formula:
[0051]
[0052] Properties: white solid;
[0053] m.p.122.5-124.7℃; optical purity: 91%ee; HPLC analysis conditions: Chiralpak OD-H (n-hexane / i-PrOH=90 / 10, 0.8mL / min), t R (minor)13.745min,t R (major) 18.205min;
[0054] Optical rotation: [α] D 20 =-127.1 (c=0.9, CH 2 Cl 2 );
[0055] 1 H NMR (400MHz, CDCl 3 )δ=8.77(s,1H),6.97–6.91(m,1H),6.91–6.87(m,1H),6.72–6.64(m,1H),6.62–6.51(m,1H),6.33–6.26( m,1H),6.21–6.14(m,1H),4.87(s,1H)...
Embodiment 3
[0058] Add 6-chloro-3-trifluoromethyl-benzoxazinone (0.05mmol), pyrrole (0.06mmol), (R)-spirocyclic phosphoric acid (0.0025mmol) shown in structural formula (1) in the reaction flask, Inject 0.5mL xylene and react at room temperature for 18 hours. After the reaction is completed, directly use silica gel column chromatography with ethyl acetate / petroleum ether=1:8 as the eluent to obtain the corresponding optically active 2H-1,4-benzene oxazin-2-one derivatives, yield 91%; product characterization as follows:
[0059] Structural formula:
[0060]
[0061] Appearance: brown solid;
[0062] m.p.131.1-133.5°C;
[0063] Optical purity: 92%ee; HPLC analysis conditions: Chiralpak OD-H (hexane / i-PrOH = 90 / 10, 0.8mL / min), t R (minor)14.620min,t R (major) 29.410min;
[0064] Optical rotation: [α] D 20 =-173.8 (c=1.2, CH 2 Cl 2 );
[0065] 1 H NMR (400MHz, CDCl 3 )δ=8.76(s,1H),6.95(d,J=4Hz,1H),6.94–6.88(m,2H),6.88–6.82(m,1H),6.32–6.26(m,1H),6.23– 6.15(m,1H),4.82(s,1H;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com