Application of stilbene glycoside to preparing analgesic medicine
A stilbene glycoside and drug technology, which is applied in the field of drugs for delaying or alleviating opioid tolerance, preparing and enhancing the analgesic effect of opioid drugs, and achieving the effects of enhancing application space, reducing opioid tolerance, and enriching sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
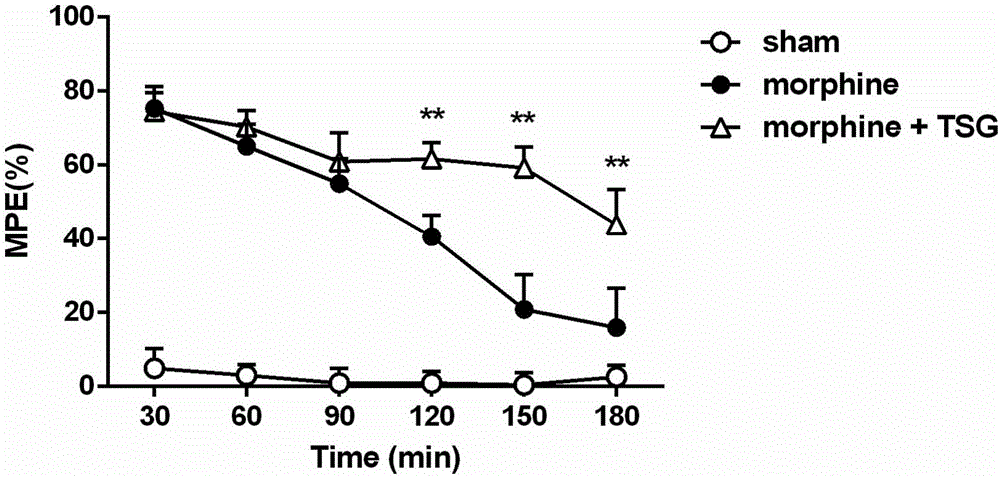
[0029] Embodiment 1 (acute analgesic effect enhancement experiment)
[0030] a) Experimental animals: healthy adult female ICR mice, clean grade, weighing 18-22 g. The experimental animals were raised in an independent environment with 12h-12h alternation of day and night, the room temperature was maintained at 24±2°C, water and food were free to drink, and the experiment was carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0031] b) Test drugs and reagents:
[0032] The drug stilbene glycoside, with a purity of 98%, is commercially available. Morphine hydrochloride was purchased from Shenyang No. 1 Pharmaceutical Factory.
[0033] c) Test method:
[0034] Dosage and method of administration of stilbene glycosides and morphine: stilbene glycosides were dissolved in physiological saline, and the administration dose was 60 mg / kg, and t...
Embodiment 2
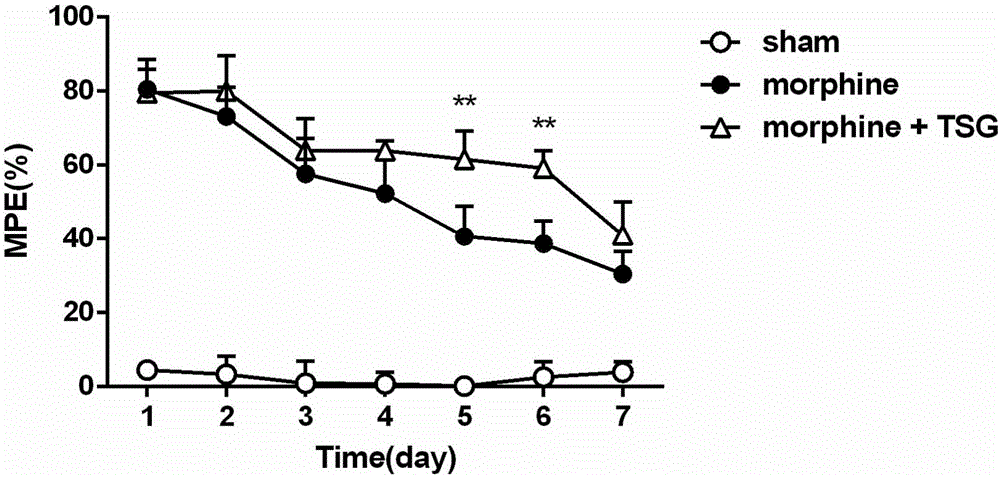
[0039] Example 2 (Opioid Tolerance Alleviation Experiment)
[0040] a) Test animals:
[0041] Female ICR mice, clean grade, weighing 18-22 g. The experimental animals were raised in an independent environment with 12h to 12h alternation of day and night, the room temperature was maintained at 24±2°C, and they had free access to water and food. Experiments were carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0042] b) Test drugs and reagents:
[0043] The drug stilbene glycoside, with a purity of 98%, is commercially available. Morphine hydrochloride was purchased from Shenyang No. 1 Pharmaceutical Factory.
[0044] c) Test method:
[0045] Dosage and method of administration of stilbene glycosides: stilbene glycosides are dissolved in physiological saline, the dose is 60 mg / kg, and the administration method is intragastric administ...
Embodiment 3
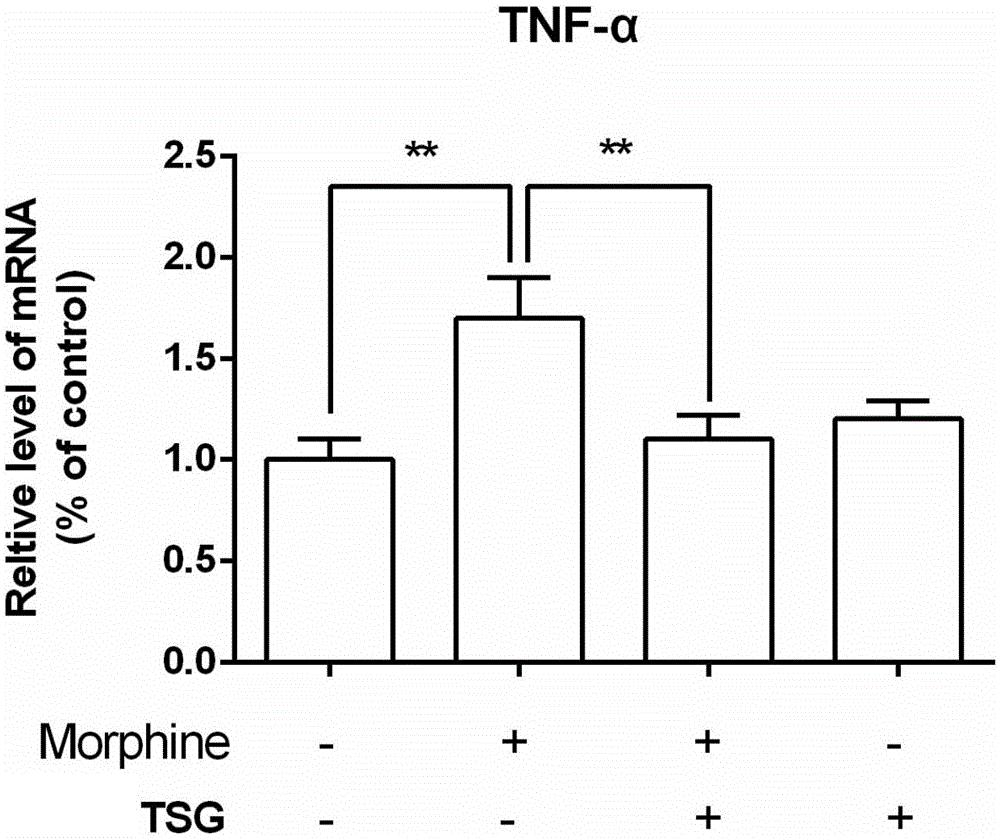
[0050] Embodiment 3 (inhibition of inflammatory factor mRNA up-regulation experiment)
[0051] a) Test cells:
[0052] BV2 microglial cells were cultured in DMEM medium containing 10% fetal bovine serum, 5% CO2, and cultured in a constant temperature incubator at 37°C. It can be subcultured when it covers 80% of the bottom of the culture bottle. 24 hours before the experiment, the medium was replaced with 0.5% fetal bovine serum high glucose DMEM.
[0053] b) Test drugs and reagents:
[0054] Fetal bovine serum was purchased from Hyclone; DMEM and Trizol were purchased from Invitrogen; TaKaRaPrimeScript kit was purchased from Dalian Bao Biological Engineering Co., Ltd.
[0055] c) Test method:
[0056] Real-time quantitative PCR: the RNA of each group was extracted by Trizol method. The reverse transcription reaction uses SYBRGreenAssay of TaKaRaPrimeScript kit, the reaction system is: 5×PrimeScriptBuffer2μl, PrimeScriptRTEnzyme0.5μl, OligodTPrimer0.5μl, Random6mers0.5μl,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com