Application of lignan compound
A technology of lignans and compounds, applied in the application field of lignans, can solve problems such as no research report on lowering blood lipids, etc., and achieve the effect of obvious blood lipid lowering effect, significant treatment, and reduced level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. Prepare (-)-eugenol-4-O-glucoside from wild pineapple
[0033] Get wild pineapple 5-10kg, decoct with water 3-10 times after pulverizing, each time with 10 times the water of volume decocting 1-6 hour (using the water of 15-50 times of volume altogether). The extracts were combined, filtered, and evaporated to dryness under reduced pressure to obtain extract (about 1000 g). Suspend the extract in 10L distilled water and extract with petroleum ether (5000ml×5-10 times). The mother liquor was extracted with n-butanol to obtain n-butanol extract. The n-butanol extract was taken and loaded onto a silica gel column (Qingdao Ocean Chemical Co., Ltd. column chromatography silica gel, particle size 60-80 μm). Different gradients of chloroform-methanol mixed solvents (chloroform:methanol=100:0-0:100, v / v) were used for elution, and the flow rate was 6-10 ml / min. The amount of mixed solvent for each gradient is 6.0 L, and fractions are collected according to the elu...
Embodiment 2
[0037] Embodiment 2. Characterization of compounds of the present invention
[0038] The structure of the tested compound is determined by its spectral data, and its spectral data are as follows:
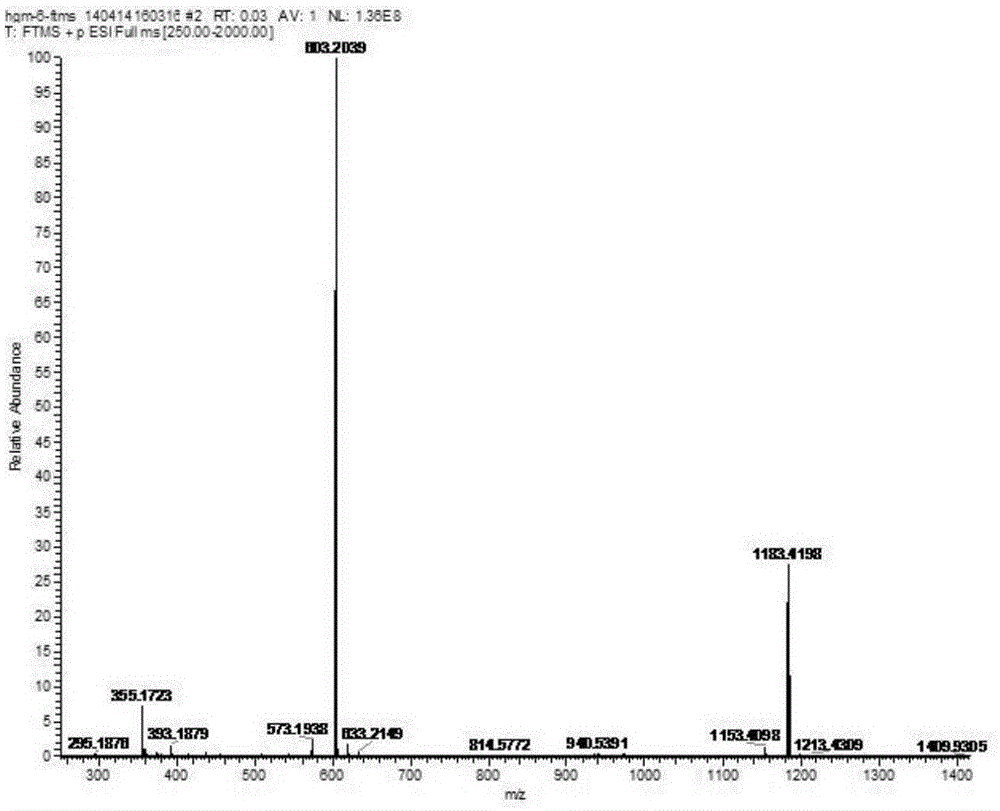
[0039] HR-ESI-MSm / z:603.2039[M+Na] + . 1H-NMR (600MHz, DMSO-d 6 )δ:6.66(2H,s,H-2,H-6),6.60(2H,s,H-2',H-6'),4.89(1H,d,J=7.2Hz,H-1” ),4.66(1H,d,J=4.2Hz,H-7'),4.61(1H,d,J=4.2Hz,H-7),4.18(2H,dd,H-9,H-9') ,3.79(2H,m,H-9,H-9'),3.76(6H,s,3,5-OCH 3 ),3.75(6H,s,3',5'-OCH 3 ),3.59(1H,dd,J=11.4,4.2Hz,H-6"),3.40(1H,m,H-6"),3.01~3.20(6H,m,H-2",H-3" ,H-4",H-5",H-8,H-8'); 13 C-NMR (150MHz, Pyr-d 6 )δ: 132.4 (C-1), 105.3 (C-2, C-6), 154.3 (C-3, C-5), 138.7 (C-4), 86.6 (C-7), 55.2 (C- 8), 72.4(C-9), 56.9(2×OCH 3 ), 57.1 (2×OCH 3 ),124.2(C-1'),105.4(C-2',C-6'),149.7(C-3',C-5'),137.7(C-4'),86.69(C-7' ), 55.3(C-8'), 72.6(C-9'), 105.2(glc-1), 776.4(glc-2), 78.7(glc-3), 72.0(glc-4), 79.0(glc- 5), 63.0 (glc-6).
[0040] Based on the spectrum data, it can be determined that the obtained compo...
Embodiment 3
[0041] Embodiment 3. In vivo hypolipidemic effect of the compound of the present invention
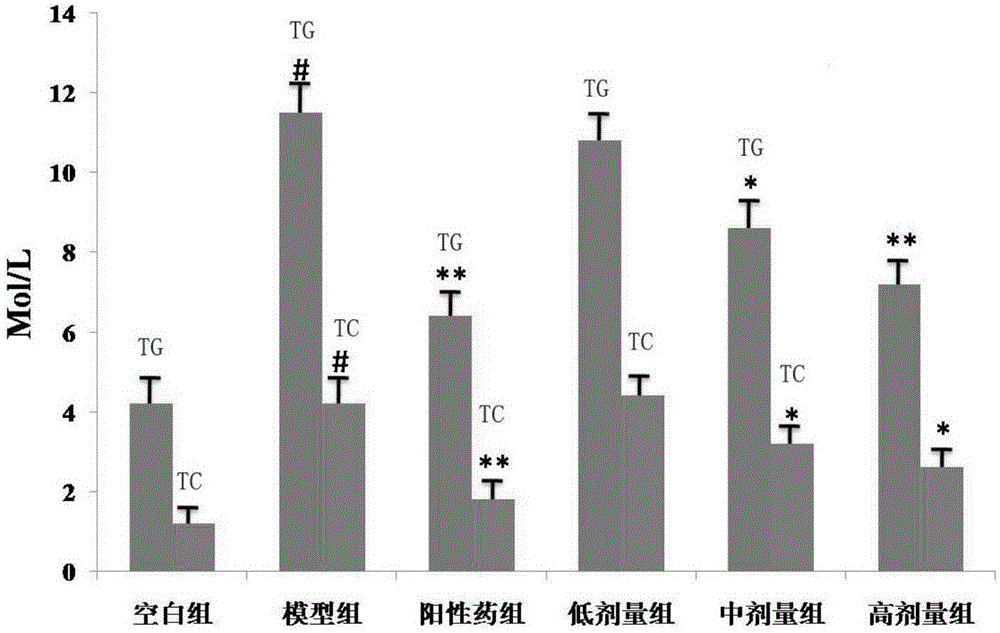
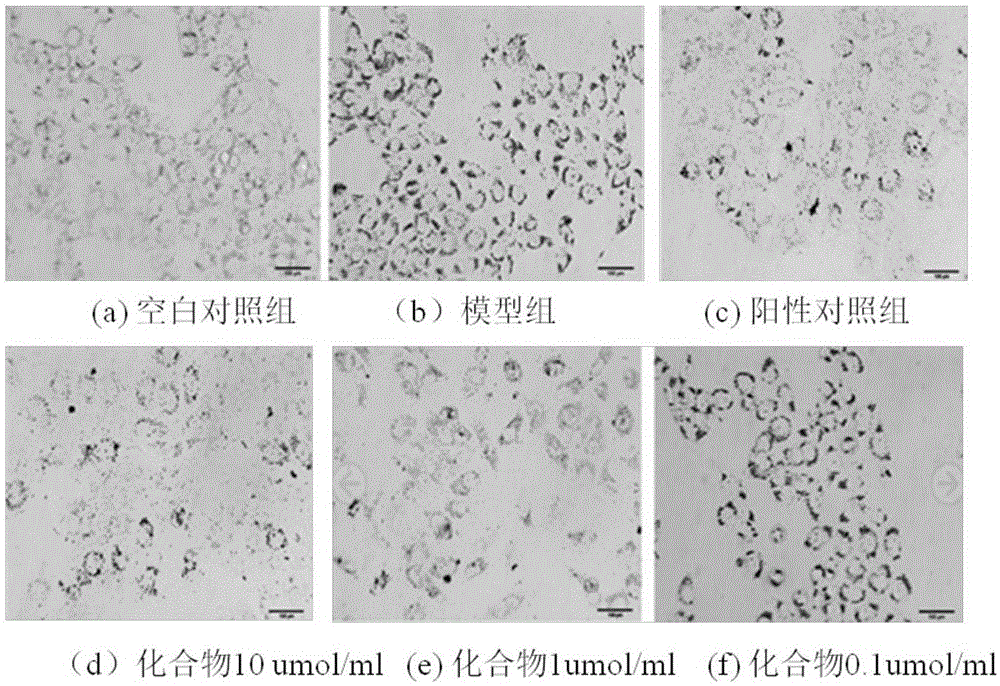
[0042] Take 60 golden hamsters of 140-160g, and randomly divide them into blank group, model group, positive drug group, and three dosage groups of compound, namely 40mg·Kg -1 , 20mg·Kg -1 , 10mg·Kg-1 ; Every group of 10, except blank group conventional feed feeding, other groups all feed high-fat feed (2.0% cholesterol, 15% lard, 0.4% bile salt, 82.6% basal feed), and positive drug group gives Simva Statin 5mg·Kg -1 , three dosage groups of compound, high, middle and low were given the compound of the present invention by intragastric administration, and the blank group and the model group were given equal volumes of tap water by intragastric administration. After 40 days of administration, the golden hamsters in each group were fasted for 12 hours, their eyeballs were removed to get blood, centrifuged at 3000r / min for 10 minutes, and the serum was taken, and the content of TC and T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com