Solid acid catalysts with dual-center ionic liquid structures and preparation method and application thereof
A technology of solid acid catalyst and ionic liquid, which is applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, carboxylate preparation, etc., which can solve the limitation of industrial application of ionic liquid and poor stability , synthesis difficulties and other problems, to achieve the effect of low cost, simple synthesis method and high synthesis purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
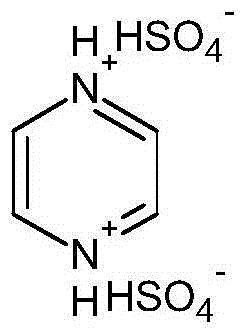
[0016] Embodiment 1: pyrazine bishydrogensulfate
[0017] Add 40.05g of pyrazine to a 250mL three-neck round bottom flask, and accurately weigh 98.08g of concentrated sulfuric acid to prepare a 50% dilute sulfuric acid aqueous solution by mass. Add it into a three-necked round-bottomed flask containing pyrazine, raise the temperature to 100°C and continue the reaction for 16 hours after the completion of the dropwise addition, and remove the water by rotary evaporation after the reaction. weight to give a tan solid. The product yield is 98%. Its chemical structural formula is:
[0018]
[0019] Characterization results: elemental analysis theoretical value C: 17.39%, H: 2.90%, N: 10.14%, measured value C: 17.42%, H: 2.98%, N: 10.10%. H NMR 1 HNMR (D 2 0, 300 MHz), δ = 8.646 (s, 4H, CH). Its melting point was measured by DSC scanning: 108.2°C.
Embodiment 2
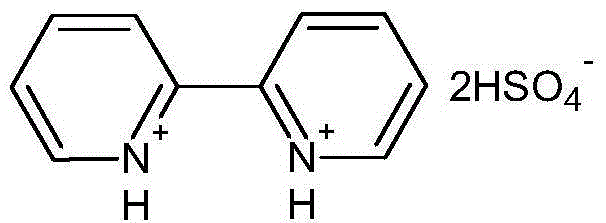
[0020] Example 2: 2,2'-bipyridyl bishydrogensulfate
[0021] Add 39.05g of 2,2'-bipyridine to a 250mL three-neck round-bottom flask, and accurately weigh 49.04g of concentrated sulfuric acid to prepare a 50% dilute sulfuric acid aqueous solution by weight. Dilute sulfuric acid was slowly added dropwise into a three-necked round-bottomed flask containing 2,2'-bipyridine. After the dropwise addition, the temperature was raised to 100°C and the reaction continued for 8 hours. After the reaction was completed, the water was removed by rotary evaporation. The obtained sample was extracted three times with benzene and ether. And vacuum-dried at 80°C to constant weight to obtain a pink solid. The product yield is 99%. Its chemical structural formula is:
[0022]
[0023] Characterization results: elemental analysis theoretical values C: 34.09%, H: 3.41%, N: 7.95%, measured values C: 34.05%, H: 3.40%, N: 7.98%. H NMR 1 HNMR (D 2 0, 300 MHz), δ=7.515 (t, 2H, CH), 7.970 (t, ...
Embodiment 3
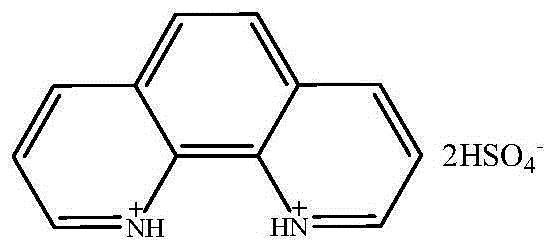
[0024] Embodiment 3: 1,10-phenanthroline dihydrogensulfate
[0025] Add 49.55g of 1,10-phenanthroline to a 250mL three-necked round-bottom flask, and accurately weigh 49.04g of concentrated sulfuric acid to prepare a 50% dilute sulfuric acid aqueous solution at room temperature. Slowly add dilute sulfuric acid into a three-necked round-bottomed flask containing 1,10-phenanthroline. After the addition, the temperature is raised to 100°C and the reaction is continued for 10 hours. After the reaction, the water is removed by rotary evaporation, and the obtained sample is repeatedly extracted with benzene and ether. three times, and dried under vacuum at 80 °C to constant weight to obtain a gray solid. The product yield is 99%. Its chemical structural formula is:
[0026]
[0027] Characterization results: elemental analysis theoretical values C: 38.30%, H: 3.19%, N: 7.45%, measured values C: 38.00%, H: 3.41%, N: 7.33%. H NMR 1 HNMR (D 2 0, 300 MHz), δ=7.407 (s, 2H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com