Carboxyl-containing difluoro monomer, preparation method of difluoro monomer and application of difluoro monomer to preparation of carboxyl-containing polyarylether
A technology of difluoromonomer and polyarylene ether, which is applied in the field of difluoromonomer and its preparation, can solve problems such as reducing mechanical properties, and achieve the effects of improving conductivity, hydrophilic performance and antifouling performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Synthetic 3-[4-(2,6-difluorobenzoyl) phenyl] propionic acid monomer
[0033] In the first step, under an argon atmosphere, add 100g (0.63mol) of 2,6-difluorobenzoic acid and 400mL of thionyl chloride to a 1000mL three-necked flask equipped with mechanical stirring, and drop 2mL of N,N-dimethyl Formamide, stirred after adding, heated to reflux of thionyl chloride, and reacted for 8 hours. After the reaction was completed, the thionyl chloride was removed by atmospheric distillation, and the colorless transparent liquid A was obtained by distillation under reduced pressure, that is, 2,6-difluorobenzene formyl chloride. 77.8g after weighing;
[0034] In the second step, under an argon atmosphere, add 31.72g (0.24mol) of anhydrous aluminum trichloride to a 1000mL three-necked flask equipped with a mechanical stirrer and a drying tube, dilute with 150mL of dichloromethane, and slowly drop it in an ice-water bath. 21g (0.11mol) of 2,6-difluorobenzoyl chloride ...
Embodiment 2
[0036] Example 2: Synthesis of biphenyl-type polyethersulfone with a carboxyl content of 20%
[0037] Put 1.1610g (0.004mol) of 3-[4-(2,6-difluorobenzoyl)phenyl]propionic acid and 4.5946 4,4'-dichlorodiphenyl sulfone into a 100mL three-necked flask equipped with mechanical stirring g (0.016mol), 3.7242g (0.02mol) of 4,4'-diphenol, 3.3170g (0.024mol) of potassium carbonate, the molar ratio of feeding is 1:4:5:6, the solvent sulfolane is 30mL, and toluene is 12mL. Azeotropic dehydrating agent. The reaction was carried out under the protection of argon, and the toluene was evaporated after adding water at 150°C for 3 hours, and the temperature was raised to 190°C for 15 hours, and the material was discharged in deionized water to obtain a white solid, which was washed with distilled water and ethanol and dried to obtain 8.2426g of a white solid.
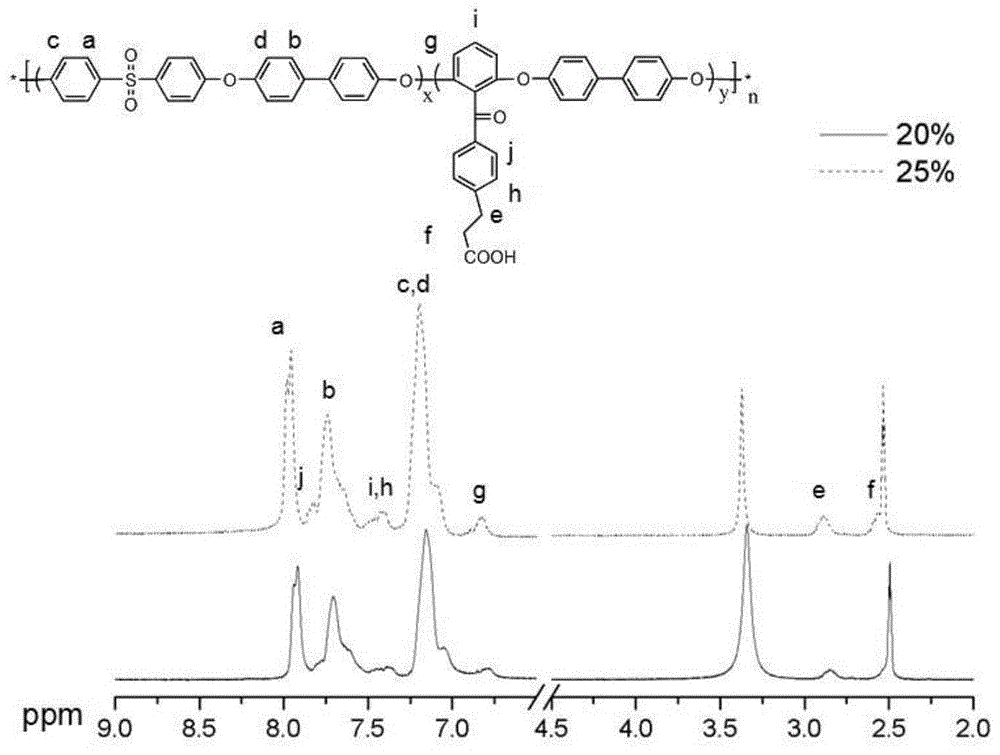
[0038] The white solid of the final product is a biphenyl type polyethersulfone with a carboxyl content of 20%, and its H NMR spectru...
Embodiment 3
[0040] Example 3: Synthesis of biphenyl-type polyethersulfone with a carboxyl content of 25%
[0041] Put 1.4513g (0.005mol) of 3-[4-(2,6-difluorobenzoyl)phenyl]propionic acid, 4.3074 g (0.015mol), 3.7242g (0.02mol) of 4,4'-diphenol, 3.3861g (0.027mol) of potassium carbonate, the molar ratio of feeding is 1:3:4:5.4, the solvent sulfolane is 30mL, and toluene is 12mL. Azeotropic dehydrating agent. The reaction was carried out under the protection of argon, with water at 150°C for 3 hours, then the toluene was distilled off, the temperature was raised to 190°C for 15 hours, and the material was discharged in deionized water to obtain a white solid, which was washed with distilled water and ethanol and dried to obtain 8.1364 g of a white solid.
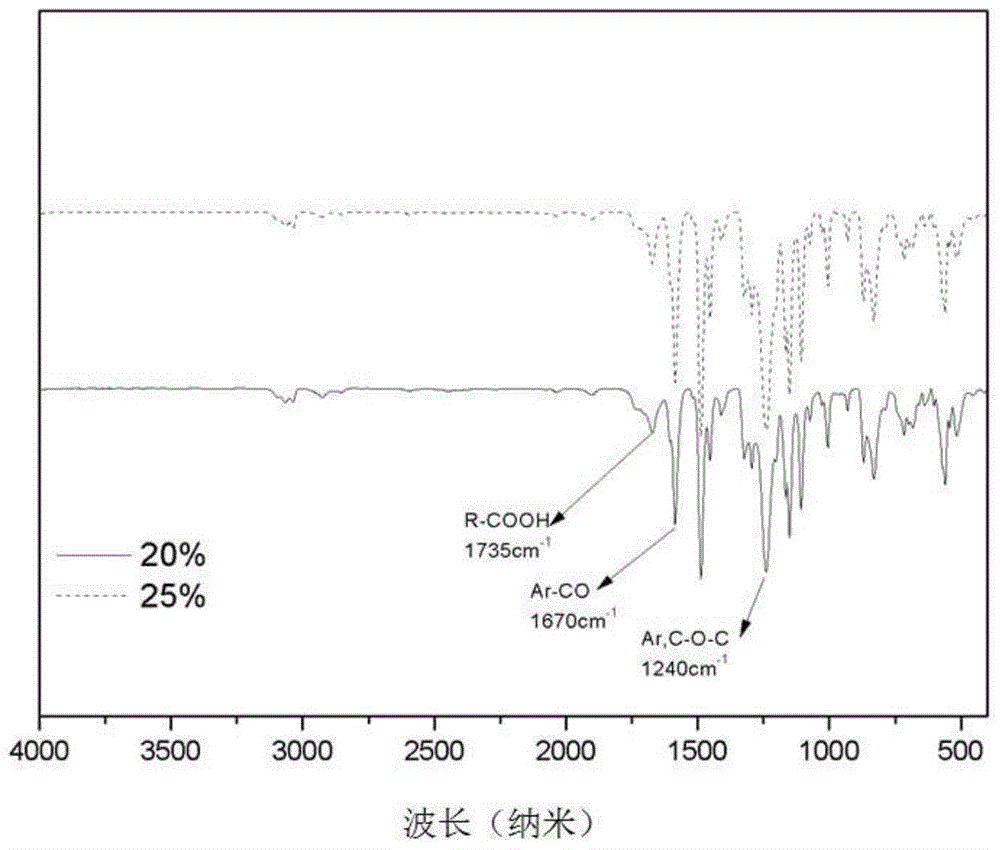
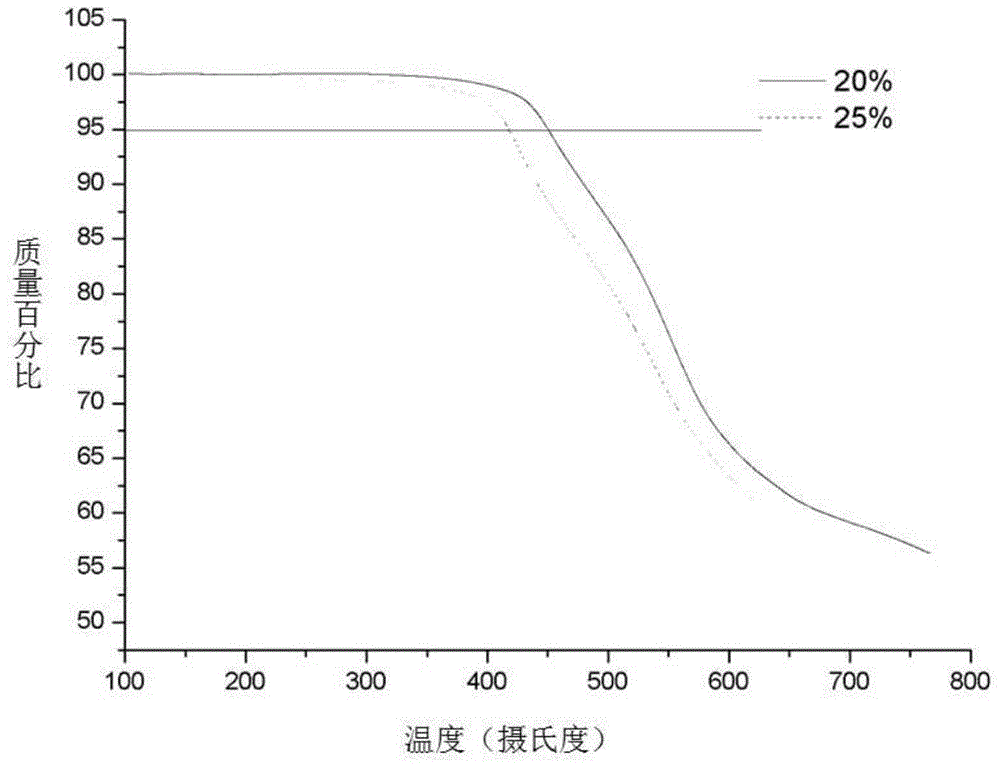
[0042] The white solid of the final product is a biphenyl type polyethersulfone with a carboxyl content of 25%. Its H NMR spectrum is shown in figure 1 , see the infrared spectrum figure 2 , see the TGA diagram image 3 , see the DS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com