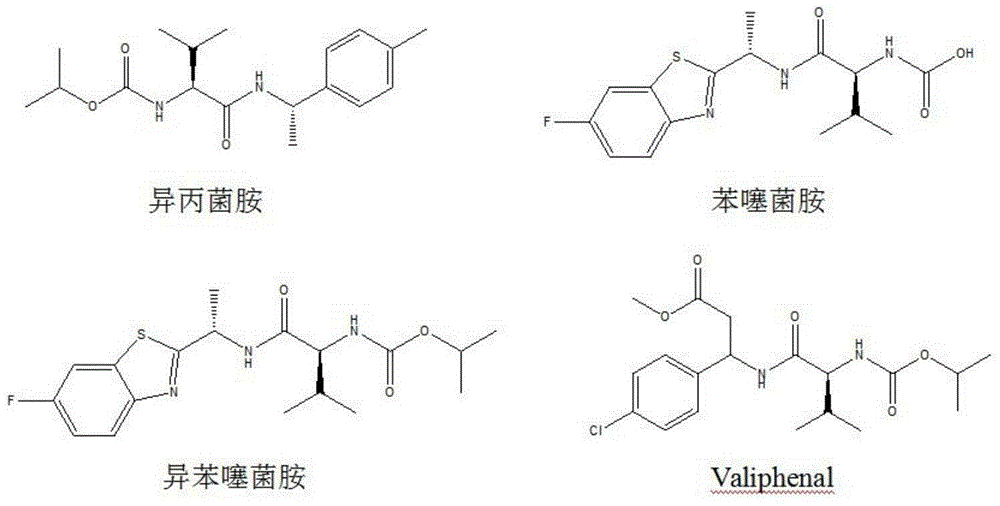
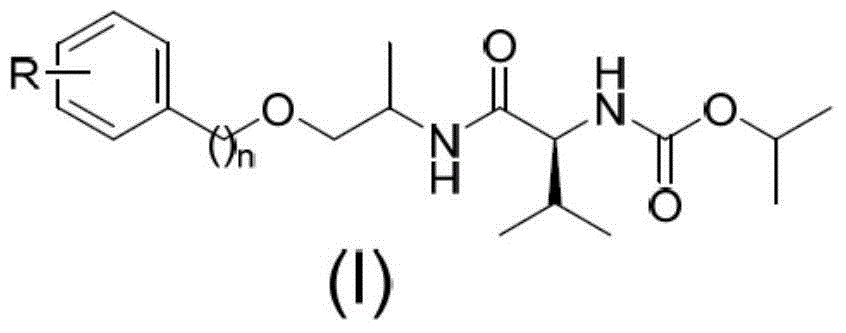
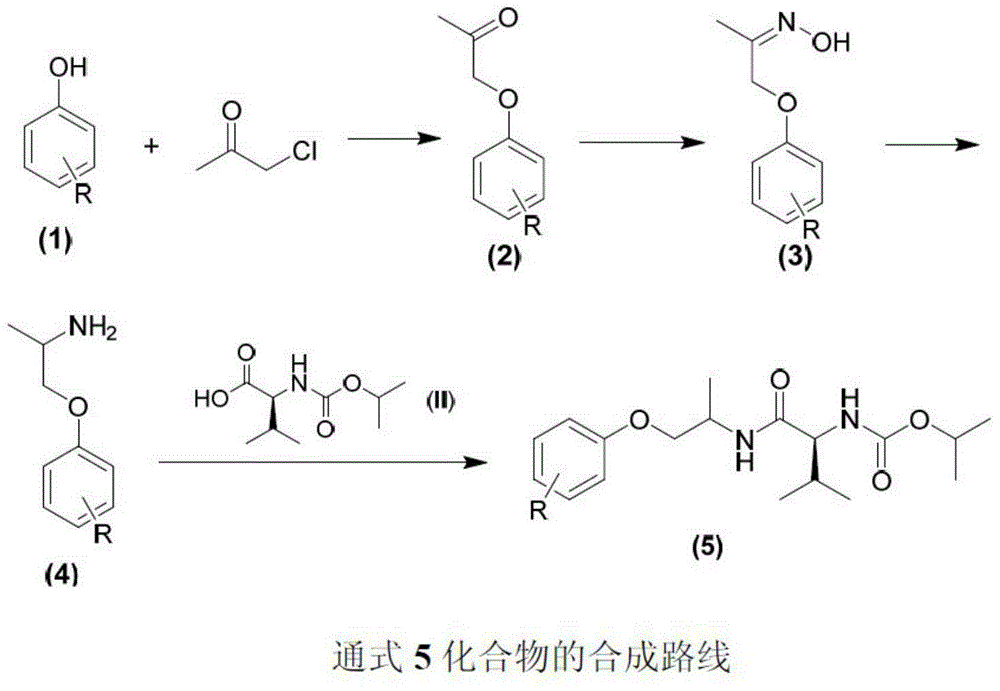
N-(1-methyl-2-substituted methyl) valine amide carbamate derivative and application
A carbamate, valine amide technology, applied in the field of pesticides, can solve the problems of short incubation period, frequent reinfection, strong destructiveness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of phenoxyacetone 2a
[0039]Phenol (3.00g, 31.88mmol) was dissolved in 50mL of acetone, anhydrous potassium carbonate (4.85g, 35.07mmol) was added, stirred at room temperature for 0.5h, potassium iodide (0.53g, 3.19mmol) was added, monochloroacetone (3.24g, 35.07mmol ) was added dropwise to the reaction solution, and refluxed for 3h. After the reaction was completed, acetone was removed by precipitation, and 50 mL of ethyl acetate was added to the residue. The organic layer was washed three times with 50 mL of saturated brine, and the water layer was backwashed once with 50 mL of ethyl acetate. The organic layers were combined and dried over anhydrous magnesium sulfate. Filtration, desolventization. Obtained 4.2 g of light yellow oily substance, yield: 87.9%. 1 HNMR (400MHz, CDCl 3 )δ7.41–7.18(m,2H,Ar-H),7.09–6.95(m,1H,Ar-H),6.91(d,J=8.1Hz,2H,Ar-H),4.55(s,2H ,CH 2 ),2.30(s,3H,CH 3 ).
[0040] According to the method of Example 1, suit...
Embodiment 2
[0041] Embodiment 2: Synthesis of 4-fluorophenoxyacetone 2b
[0042] Pale yellow oil, 1 HNMR (300MHz, CDCl 3 )δ7.05–6.88(m,2H,Ar-H),6.88–6.73(m,2H,Ar-H),4.51(s,2H,CH 2 ),2.26(s,3H,CH 3 ).
Embodiment 3
[0043] Example 3: Synthesis of 2-fluorophenoxyacetone 2c
[0044] Pale yellow oil, 1 HNMR (300MHz, CDCl 3 )δ7.21–6.75(m,4H,Ar-H),4.61(s,2H,CH 2 ),2.32(s,3H,CH 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com