Kit for screening spinal muscular atrophy virulence gene carrier and application of kit
A spinal muscular atrophy and pathogenic gene technology, applied in DNA/RNA fragmentation, DNA preparation, recombinant DNA technology, etc., can solve the problems of poor reliability, complicated operation, low accuracy, etc., and achieve good reliability and repeatability. Good, accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Primers and probes for screening gene carriers of spinal muscular atrophy and their screening methods
[0066] (1) According to the SMN1exon7 gene sequence design, primers and fluorescent probes were synthesized, and all primer / probe combinations were synthesized by Applied Biosystems, USA.
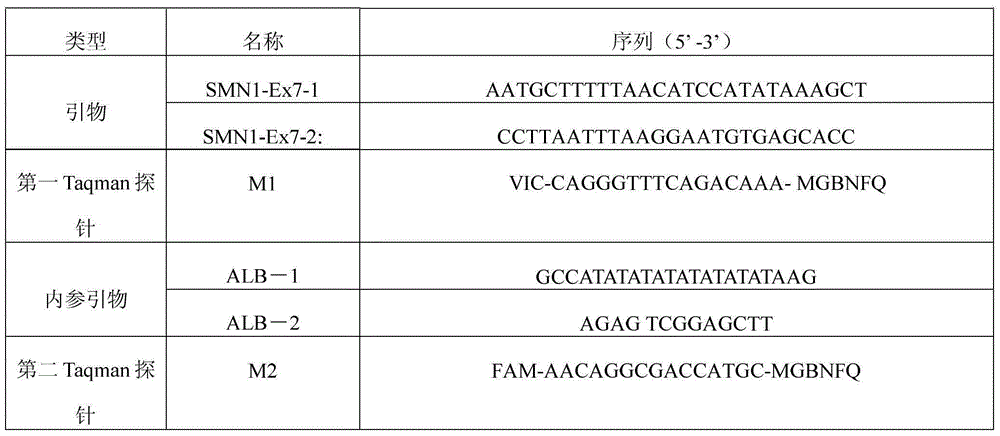
[0067] Table 1 Primers and probes used in Real-TimePCR, internal reference primers and probes
[0068]
[0069] In this example, VIC is used as the fluorophore of the first Taqman probe (SMN1Taqman probe), FAM is used as the fluorophore of the second Taqman probe (ALBTaqman probe), and MGBNFQ is used as the quencher. Its sequence is as follows:
[0070] The first Taqman probe sequence: 5'-VIC-CAGGGTTTCAGACAAA-MGBNFQ-3';
[0071] The second Taqman probe sequence is: 5'-FAM-AACAGGCGACCATGC-MGBNFQ-3';
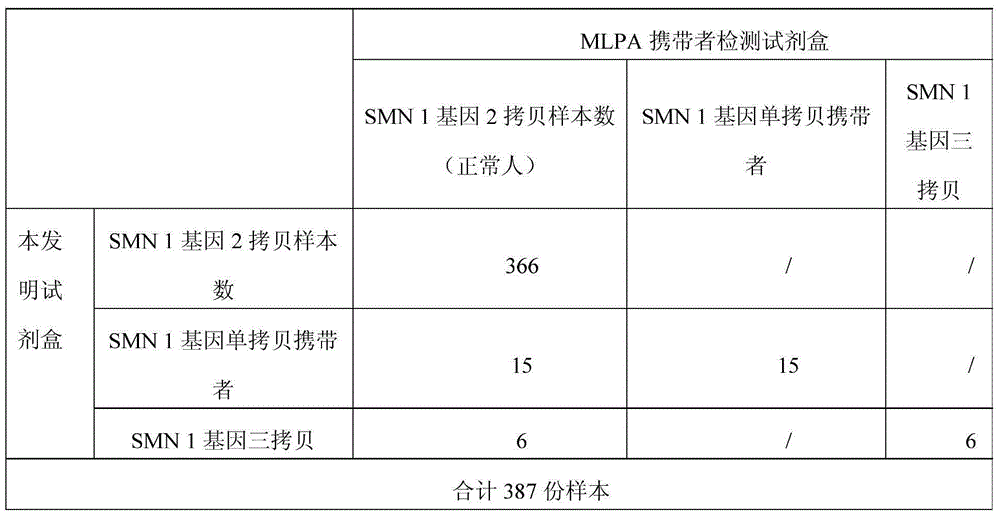
[0072] (2) Nucleic acid extraction: use QIAampbloodminikit (Qiagen Company), refer to the kit instructions for specific operations, extract gDNA from 200 μL of maternal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com