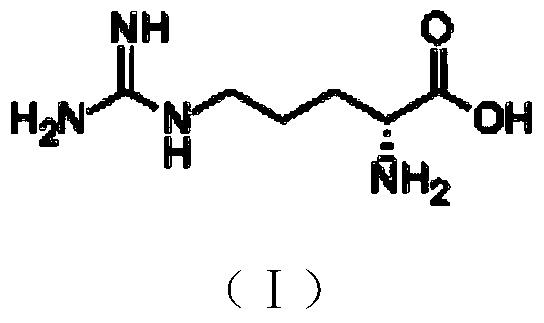
A kind of preparation method of d-arginine hydrochloride
A technology of arginine hydrochloride and arginine, which is applied in the field of preparation of D-arginine hydrochloride, can solve the problems of low yield, low product yield, high cost, etc., and achieve high yield and high preparation The method is simple and the effect is convenient to produce
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: a kind of preparation method of D-arginine hydrochloride, it comprises the following steps:
[0025] S1. Racemization: the purity is greater than 97% and L-arginine hydrochloride is dissolved in glacial acetic acid, adding catalyst salicylaldehyde, and the weight ratio of L-arginine hydrochloride, glacial acetic acid and salicylaldehyde is 1: 4:0.1, reflux at 95°C for 6 hours to obtain DL-arginine hydrochloride;
[0026] S2. Acetylation: Dissolve DL-arginine hydrochloride in sodium hydroxide and add acetic anhydride dropwise. The weight ratio of DL-arginine hydrochloride, sodium hydroxide and acetic anhydride is 1:4 : 0.5, reacted for 1.5h, cooled and crystallized, and the gained solid was AC-DL-arginine;
[0027] S3. Enzymolysis: add acylase to AC-DL-arginine, adjust the pH value to 8 with 10% sodium hydroxide, and the weight ratio of AC-DL-arginine to acylase is 1:0.02,35 Incubate at ℃ for 40 hours to obtain D-arginine hydrochloride;
[0028] S4. Refi...
Embodiment 2
[0029] Embodiment 2: a kind of preparation method of D-arginine hydrochloride, it comprises the following steps:
[0030] S1. Racemization: the purity is greater than 97% and L-arginine hydrochloride is dissolved in glacial acetic acid, adding catalyst salicylaldehyde, and the weight ratio of L-arginine hydrochloride, glacial acetic acid and salicylaldehyde is 1: 7:0.2, reflux at 105°C for 8 hours to obtain DL-arginine hydrochloride;
[0031] S2. Acetylation: Dissolve DL-arginine hydrochloride in sodium hydroxide, drop acetic anhydride, the weight ratio of DL-arginine hydrochloride, sodium hydroxide and acetic anhydride is 1:5 : 1, reacted for 3h, cooled and crystallized, and the gained solid was AC-DL-arginine;
[0032] S3. Enzymolysis: add acylase to AC-DL-arginine, adjust the pH value to 9 with 10% sodium hydroxide, and the weight ratio of AC-DL-arginine to acylase is 1:0.05, 40 Incubate at ℃ for 48 hours to obtain D-arginine hydrochloride;
[0033] S4. Refining: heat th...
Embodiment 3
[0034] Embodiment 3: a kind of preparation method of D-arginine hydrochloride, it comprises the following steps:
[0035] S1. Racemization: the purity is greater than 97% and L-arginine hydrochloride is dissolved in glacial acetic acid, adding catalyst salicylaldehyde, and the weight ratio of L-arginine hydrochloride, glacial acetic acid and salicylaldehyde is 1: 5:0.15, reflux at 100°C for 7 hours to obtain DL-arginine hydrochloride;
[0036] S2. Acetylation: Dissolve DL-arginine hydrochloride in sodium hydroxide and add acetic anhydride dropwise. The weight ratio of DL-arginine hydrochloride, sodium hydroxide and acetic anhydride is 1:4.3 : 0.8, reacted 2h, cooled and crystallized, and the gained solid was AC-DL-arginine;
[0037] S3. Enzymolysis: add acylase to AC-DL-arginine, adjust the pH value to 8- with 10% sodium hydroxide, the weight ratio of AC-DL-arginine to acylase is 1:0.03, Incubate at 37°C for 42 hours to obtain D-arginine hydrochloride;
[0038] S4. Refining...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com