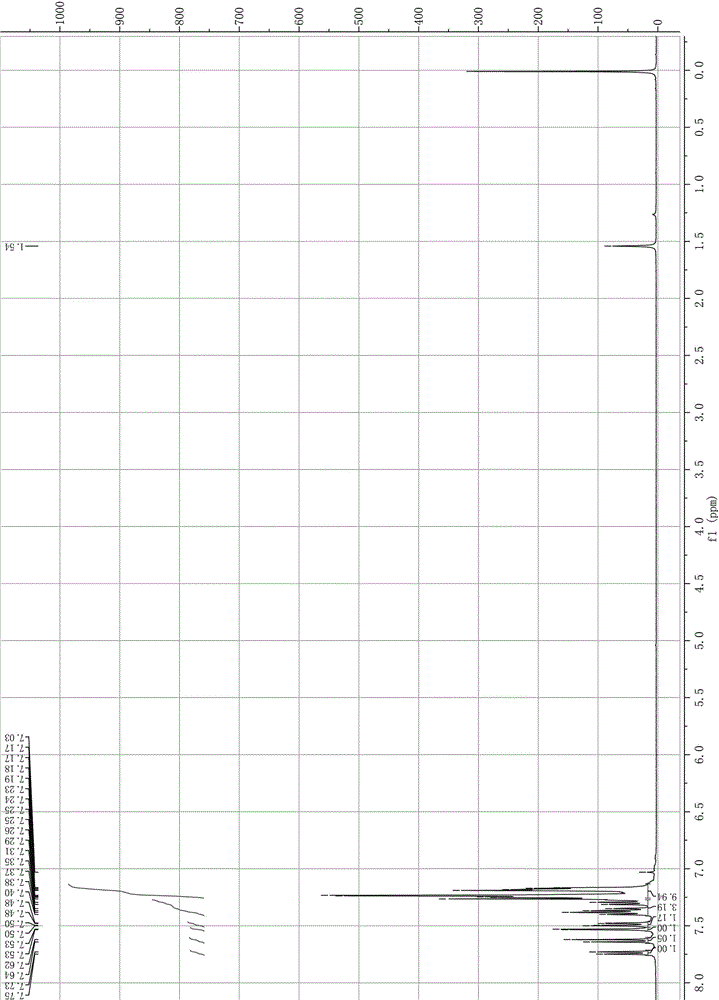
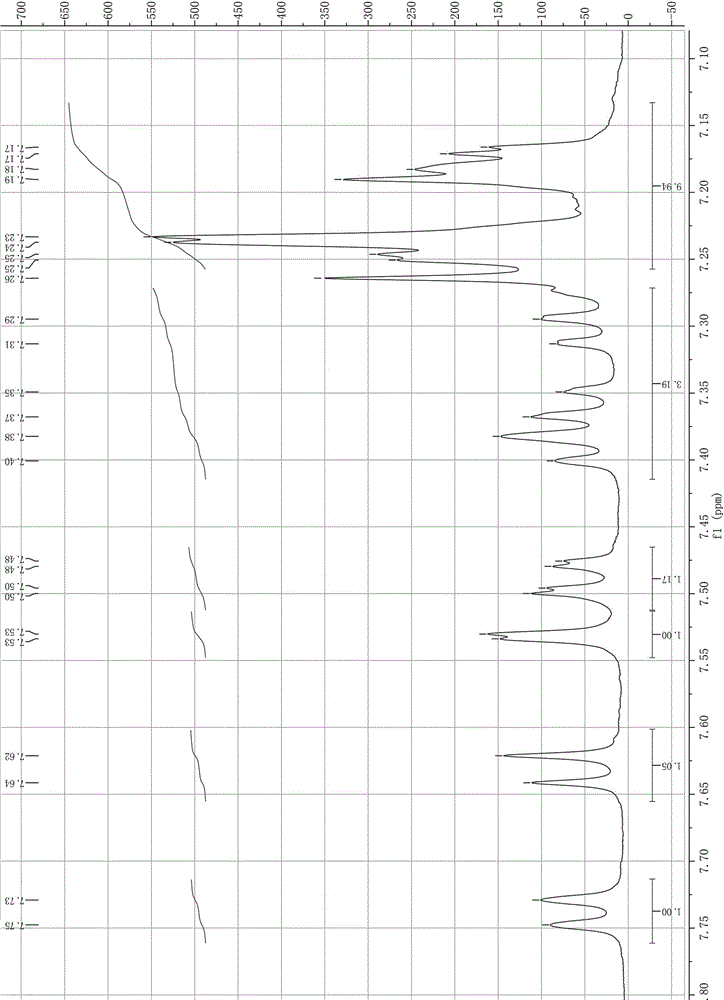
Synthesis method of 2-bromo-9,9-diphenylfluorene
A technology of diphenylfluorene and synthesis method, which is applied in the fields of chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of expensive trifluoromethanesulfonic acid, difficult post-processing, high cost, etc., and achieve good catalytic effect , product yield improvement, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic method of 2-bromo-9,9-diphenylfluorene includes the following steps:
[0026] (1) Mix 10mmol 2-bromo-9-phenyl-fluorene-9-alcohol with 100mmol benzene, heat up to 61℃ with stirring, and pass in hydrogen chloride gas until TLC monitors the raw material point (2-bromo-9-phenyl -Fluorene-9-alcohol) has basically disappeared. After the aeration is completed, the reaction system is heated to the reflux temperature, refluxed for 1 hour, and the water in the system is separated by a water separator while refluxing;
[0027] (2) Cool the system to 17±2°C, add the catalyst in batches within 0.5h, after the addition, heat up to 35°C and react for 3.5h; the catalyst consists of 10mmol anhydrous aluminum trichloride and 1mmol anhydrous trichloride It is composed of iron, the catalyst is added in three batches, and the weight ratio of the three additions is 3:2:1;
[0028] (3) Separate and purify to obtain 2-bromo-9,9-diphenylfluorene. The separation and purification operatio...
Embodiment 2
[0030] The synthetic method of 2-bromo-9,9-diphenylfluorene includes the following steps:
[0031] (1) Mix 10mmol of 2-bromo-9-phenyl-fluorene-9-ol and 180mmol of benzene. The temperature is raised to 66°C with stirring, and hydrogen iodide gas is introduced until the raw material point disappears after TLC monitoring. After the ventilation is completed, The reaction system is heated to the reflux temperature, and the reflux is completed for 1.5 hours, and the water in the system is separated by a water separator while refluxing;
[0032] (2) Cool the system to 18±2℃, add the catalyst in batches within 0.5h. After the addition is completed, the temperature is raised to 50℃ and the reaction ends after 4.5h; the catalyst consists of 12mmol anhydrous aluminum trichloride and 3mmol anhydrous trichloride It is composed of iron, the catalyst is added in three batches, and the weight ratio of the three additions is 3:2:1;
[0033] (3) Separate and purify to obtain 2-bromo-9,9-diphenylfluor...
Embodiment 3
[0035] The synthetic method of 2-bromo-9,9-diphenylfluorene includes the following steps:
[0036] (1) Mix 10mmol 2-bromo-9-phenyl-fluorene-9-alcohol and 170mmol benzene, heat up to 64℃ with stirring, and pass in enough hydrogen bromide gas until TLC monitors that the raw material point has almost disappeared and the ventilation is complete Afterwards, the reaction system was heated to the reflux temperature, and the reflux was completed for 2 hours, and the water in the system was separated by a water separator while refluxing;
[0037] (2) Cool the system to 20±2℃, add the catalyst in batches within 1h. After the addition is completed, the temperature is raised to 45℃ and the reaction is finished after 5h; the catalyst consists of 12mmol anhydrous aluminum trichloride and 3mmol anhydrous iron trichloride Composition, the catalyst is added in three batches, and the weight ratio of the three additions is 3:2:1;
[0038] (3) Separate and purify to obtain 2-bromo-9,9-diphenylfluorene....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com