Polypeptide capable of specifically targeting HER2 protein and application of polypeptide
A kind of specific and targeted technology, applied in peptides, peptides specifically targeting HER2 protein and its application fields, can solve the problems of poor targeting effect and insufficient interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 The synthesis of polypeptide of the present invention
[0055] 1) Experimental instruments and materials
[0056] Dimethylformamide (DMF), piperidine, resin, dichloromethane (DCM), ninhydrin reagent (ninhydrin, vitamin C, phenol), tetramethyluronium hexafluorophosphate (HBTU), six Hydropyridine (piperidine), triisopropylsilane (TIS), ethanedithiol (EDT), anhydrous ether, trifluoroacetic acid (TFA), N-methylmorpholine (NMM), methanol, various amino acids , Peptide solid-phase synthesis tube.
[0057] 2) Solution preparation
[0058] Deprotection solution - hexahydropyridine: DMF = 1:4
[0059] Reaction solution——NMM:DMF=1:24
[0060] Lysis solution - TFA (92.5%), TIS (2.5%), EDT (2.5%), H 2 O(2.5%)
[0061] Ninhydrin Test Solution—Ninhydrin: Vitamin C: Phenol = 1:1:1
[0062] 3) Experimental steps
[0063]Weigh the resin and put it into the peptide solid-phase synthesis tube (hereinafter referred to as the reactor), and add an appropriate amount of D...
experiment example 1
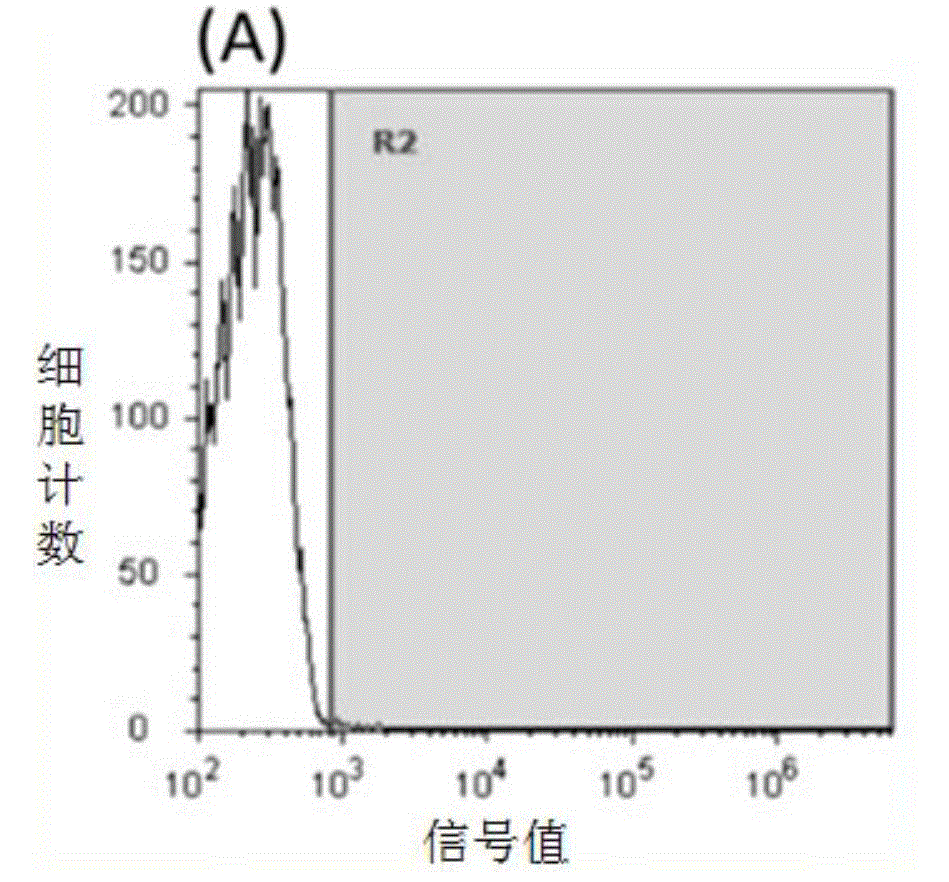
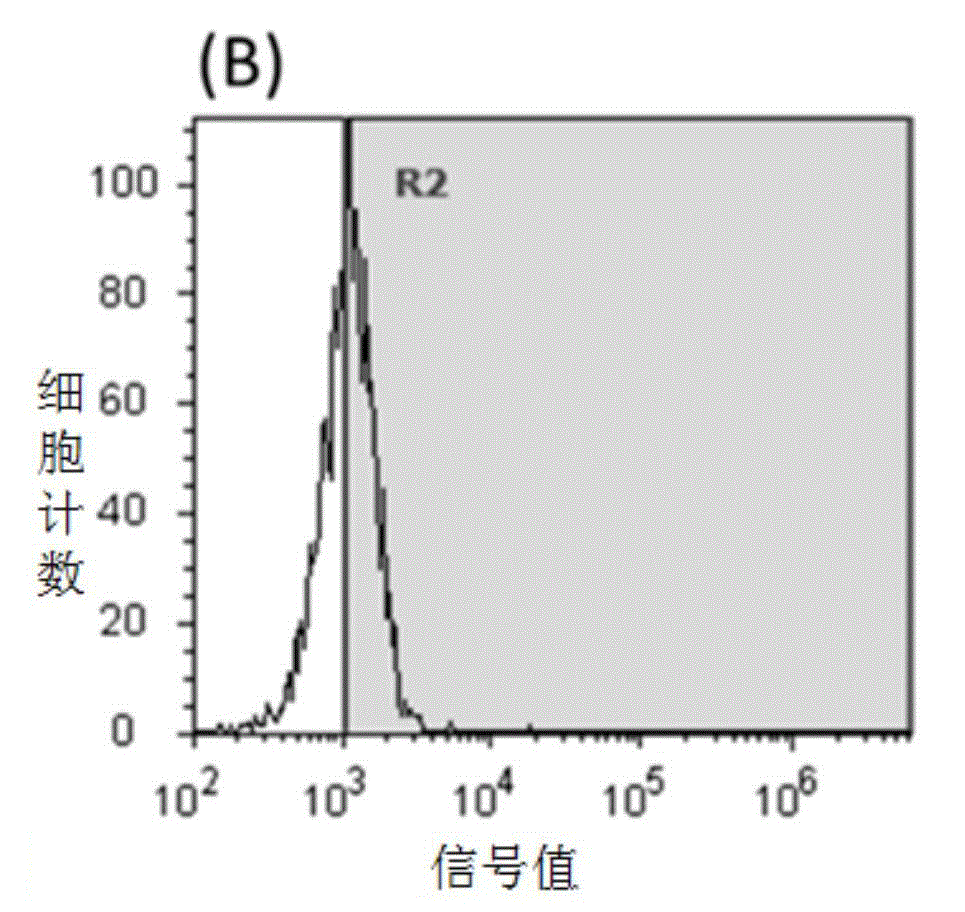
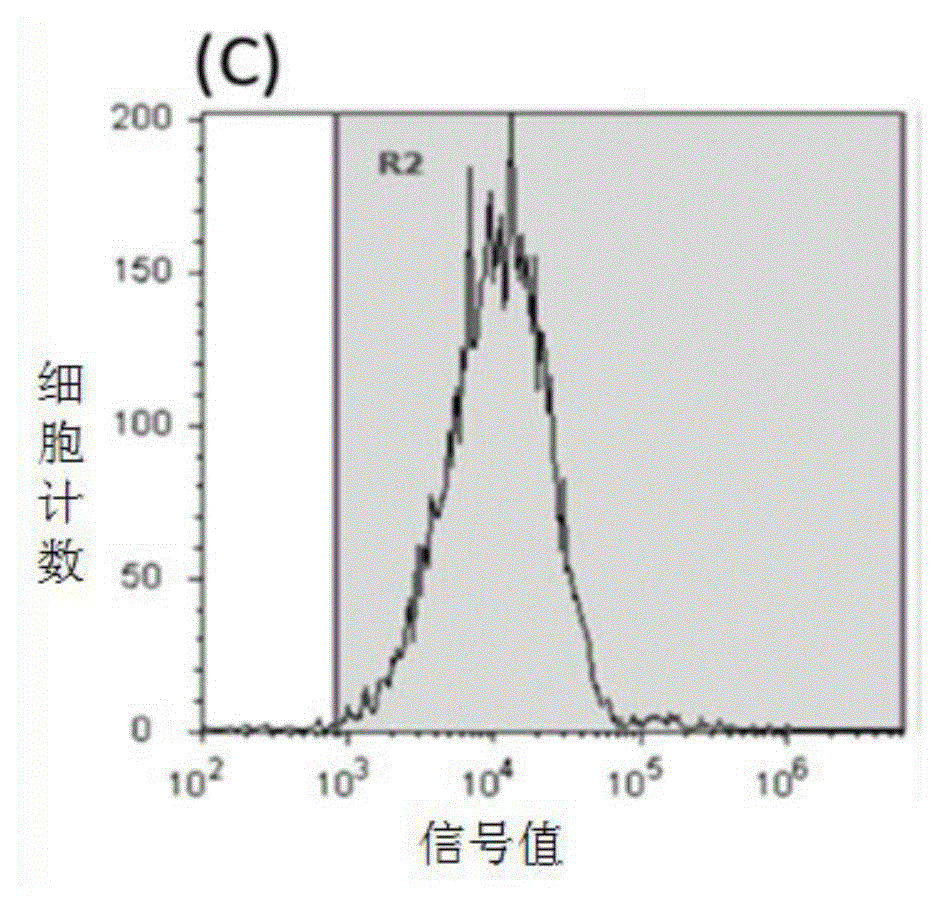
[0071] Experimental Example 1 Flow cytometry detection of the binding effects of SEQIDNO.1, SEQIDNO.2, SEQIDNO.3, and SEQIDNO.4 on human HER2-positive breast cancer cells respectively
[0072] 1. Experimental method
[0073] Collect human breast cancer HER2 high expression cell line SKBR3, suspend in RPMI1640 culture medium containing 10% heat-inactivated fetal bovine serum, cell density is 1x10 6 / mL, divided into four 1.5mL EP tubes, 200μL / tube. Add fluorescein isothiocyanate (FITC) labeled SEQIDNO.1, SEQIDNO.2, SEQIDNO.3, SEQIDNO.4 and anti-HER2 antibody (ebioscience) respectively, the final concentration is 50μM / L, the control group uses the same amount of polypeptide 0.01mMPBS (phosphate buffer pH 7.4) was used instead of the polypeptide. After incubating in an ice bath in the dark for 30 minutes, centrifuge at 1000g for 4 minutes to collect cells, add 1mL PBS to wash, repeat washing 3 times, add 500μL PBS, mix well, and use flow cytometry to detect fluorescence intensi...
experiment example 3
[0081] Experimental Example 3 Immunofluorescence method to detect the binding effects of SEQIDNO.1, SEQIDNO.2, SEQIDNO.3, and SEQIDNO.4 on human HER2-positive breast cancer cells respectively
[0082] 1. Experimental method
[0083] The human breast cancer HER2 high-expressing cell line SKBR3 was suspended in RPMI1640 culture medium containing 10% heat-inactivated fetal bovine serum, and seeded in three confocol small dishes at a density of 3000-5000 cells / dish. After cultivating for 24 hours, aspirate the medium in the small dish, and then add the culture medium containing 50 μM mol / L of fluorescein isothiocyanate (FITC) labeled SEQIDNO.1, SEQIDNO.2, SEQIDNO.3, SEQIDNO.4 polypeptide respectively The base was 200 μL, and the control group used the medium containing PBS (phosphate buffer pH 7.4) equal to the amount of the polypeptide, and the nuclei were stained with the hoechst reagent, which was diluted 1:200. Incubate in an ice bath protected from light for 30 minutes. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com