Vortioxetine semi-hydrochloride, preparation method therefor, and pharmaceutical composition thereof
A technology of vortioxetine and hydrochloride, applied in the field of vortioxetine hemihydrochloride and its preparation, can solve the problems of poor drug stability, easy mutual conversion, unsuitability for preparation development and the like, and achieves stable crystal form, The effect of high crystallinity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 4 mL of ethanol solution containing 0.1 g of vortioxetine to one side of the communicating H tube; add 1 mL of concentrated hydrochloric acid with a mass fraction of 38% to the other side of the H tube, and continue to add 3 mL of ethanol. Close the H tube to slowly diffuse the hydrogen chloride gas in the H tube, and obtain white crystals after 72 hours.
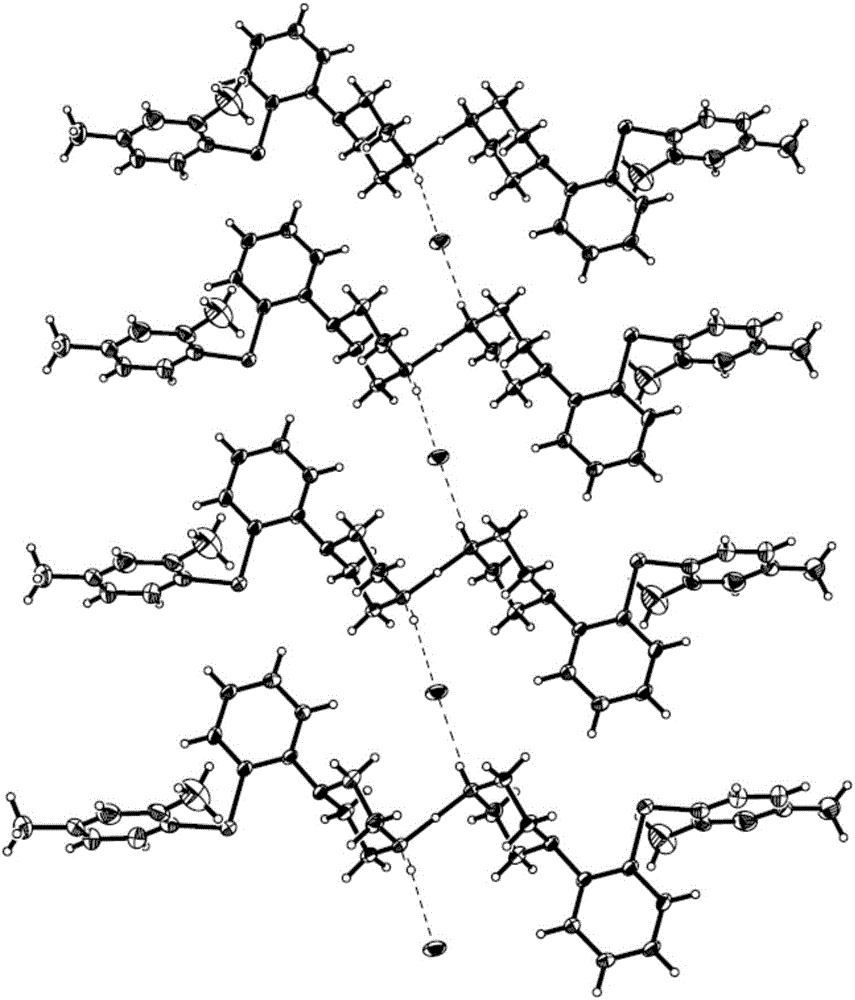
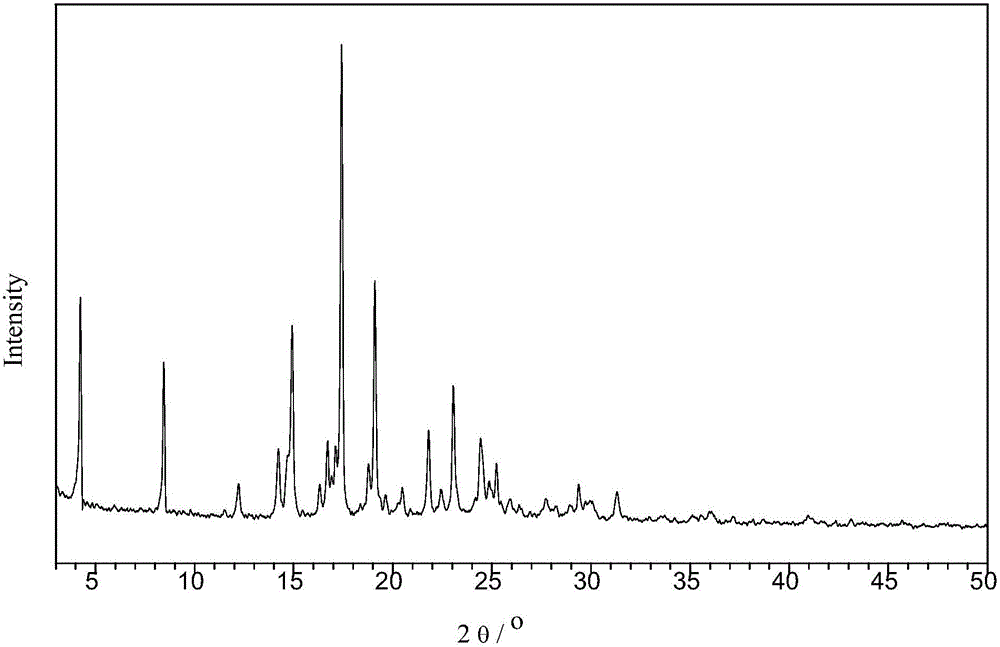
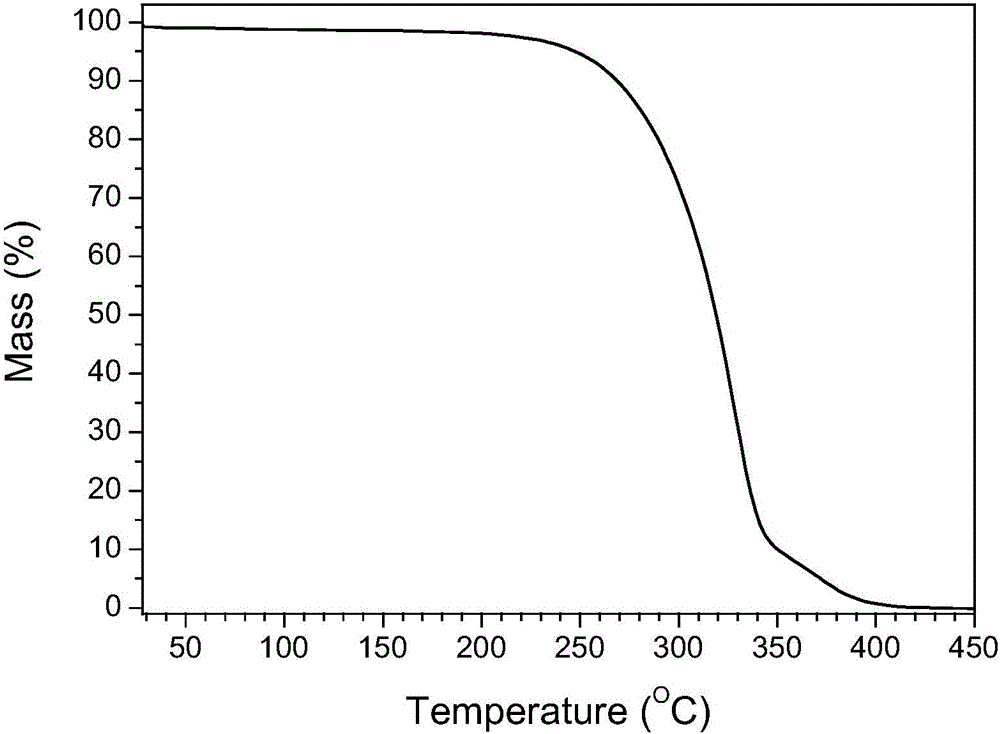
[0044] The schematic diagram of the structural unit of the crystal is shown in figure 1 As shown, the powder XRD pattern is as follows figure 2 As shown, the TG diagram is shown as image 3 As shown, the IR diagram is as Figure 4 as shown, 1 H-NMR such as Figure 5 Shown (* represents the characteristic peak of solvent).
Embodiment 2
[0046] Take 10g of vortioxetine and add it to 350mL of acetonitrile, heat and stir to dissolve it completely, and then inject hydrogen chloride gas into the container under stirring until no more weight is added, filter the solid with suction at room temperature, wash with 10mL of acetonitrile and dry to constant weight , Vortioxetine hemihydrochloride white powder 9.8g, the powder diffraction of this solid is consistent with formula (I) Vortioxetine hemihydrochloride.
[0047] 1 H-NMR (d 6-DMSO,δppm):7.33(d,1H),7.24(s,1H),7.07-7.14(m,3H),6.87-7.01(m,1H),6.40(d,1H),3.04(s,8H ), 2.32(s,3H), 2.24(s,3H).
Embodiment 3
[0049] Take 10g of vortioxetine and add it to 250mL of methanol, stir to dissolve it completely, and then inject hydrogen chloride gas into the container until the weight is no longer increased under stirring, filter the solid with suction at room temperature, wash with 10mL of methanol and dry to constant weight. Vortioxetine hemihydrochloride white powder was 9.3 g, and the powder diffraction and thermogravimetric analysis of the solid was consistent with formula (I) vortioxetine hemihydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com