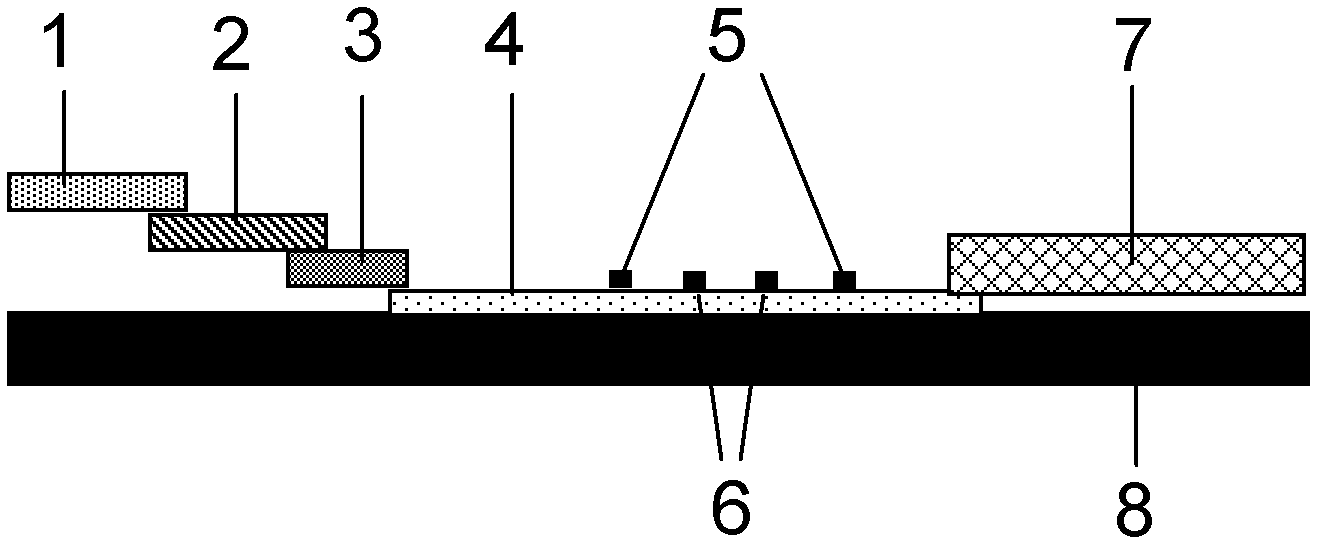
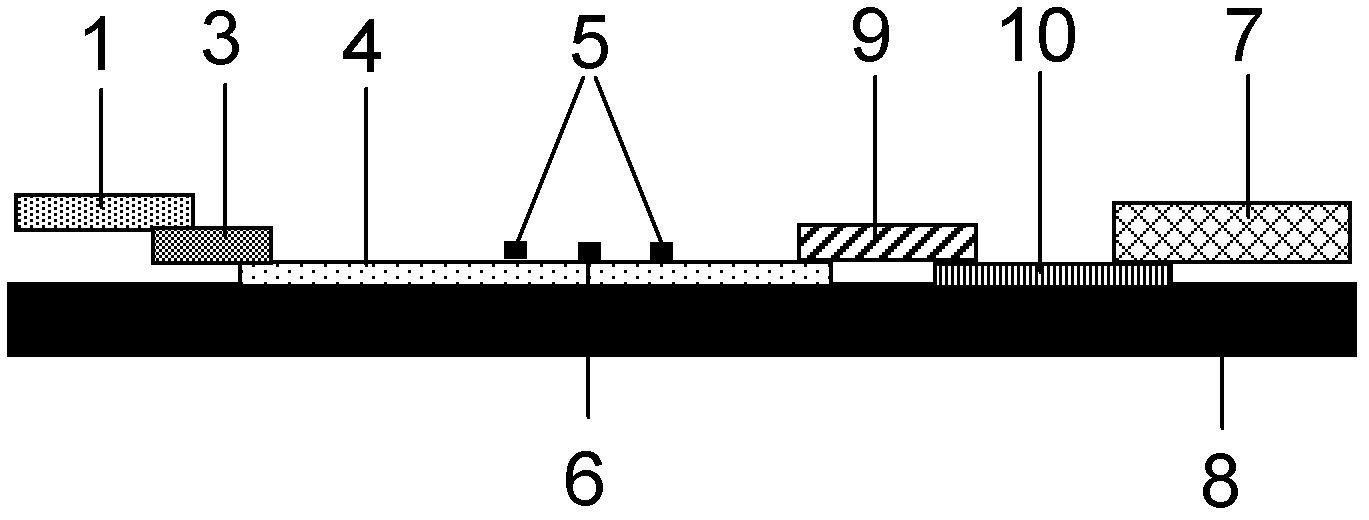
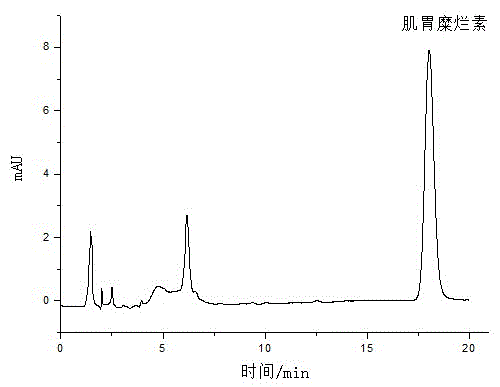
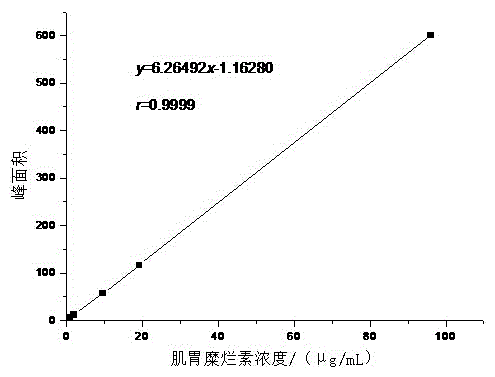
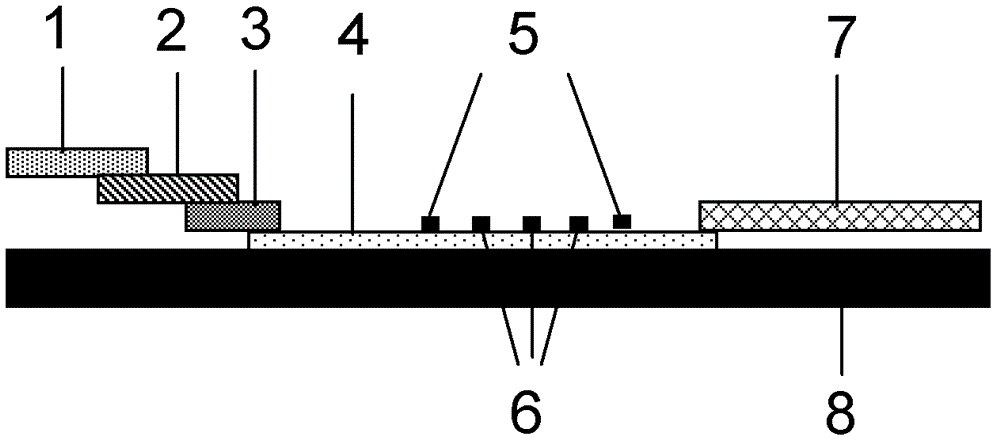
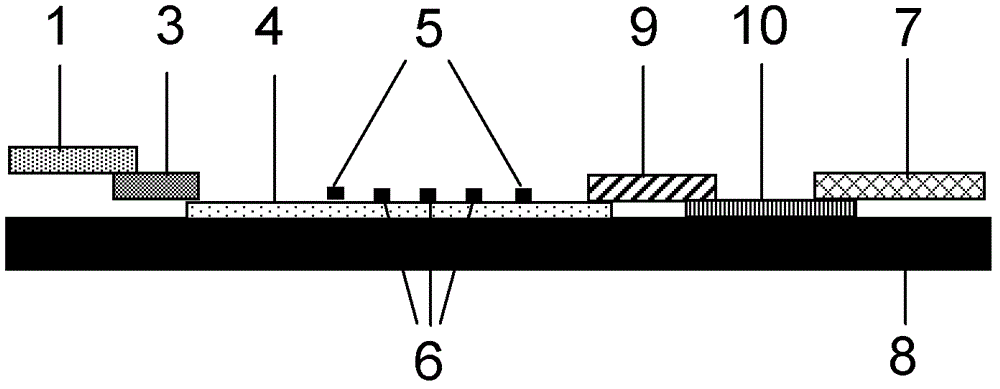
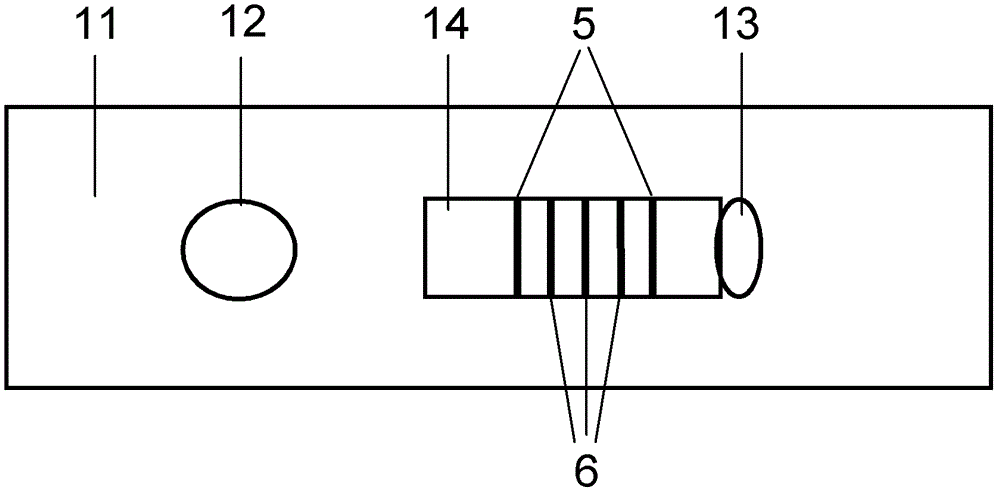
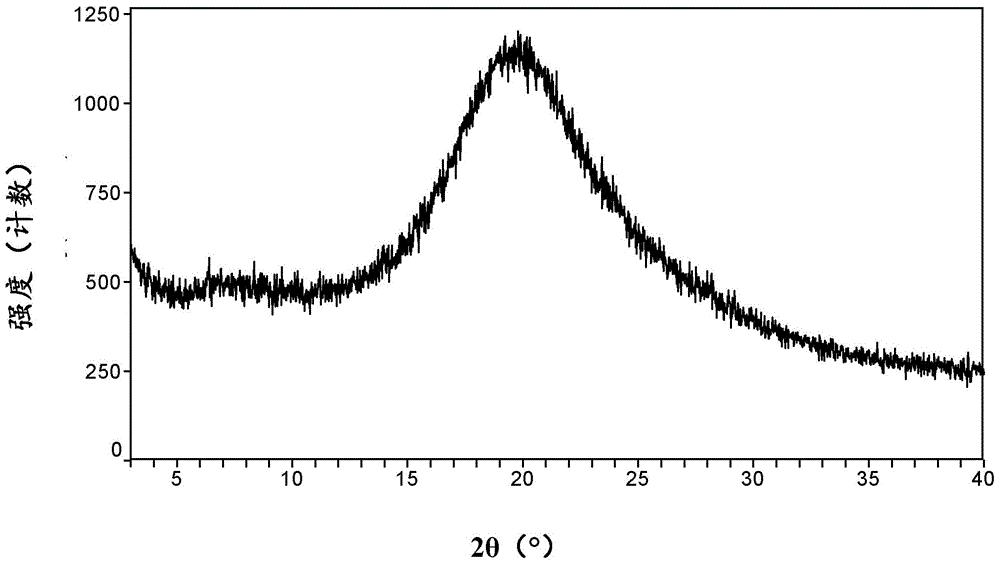
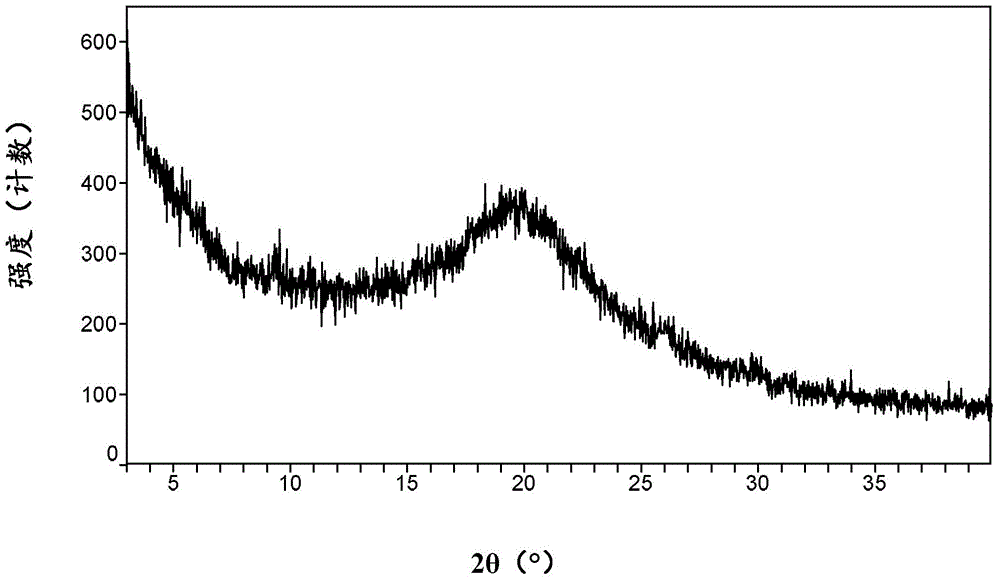
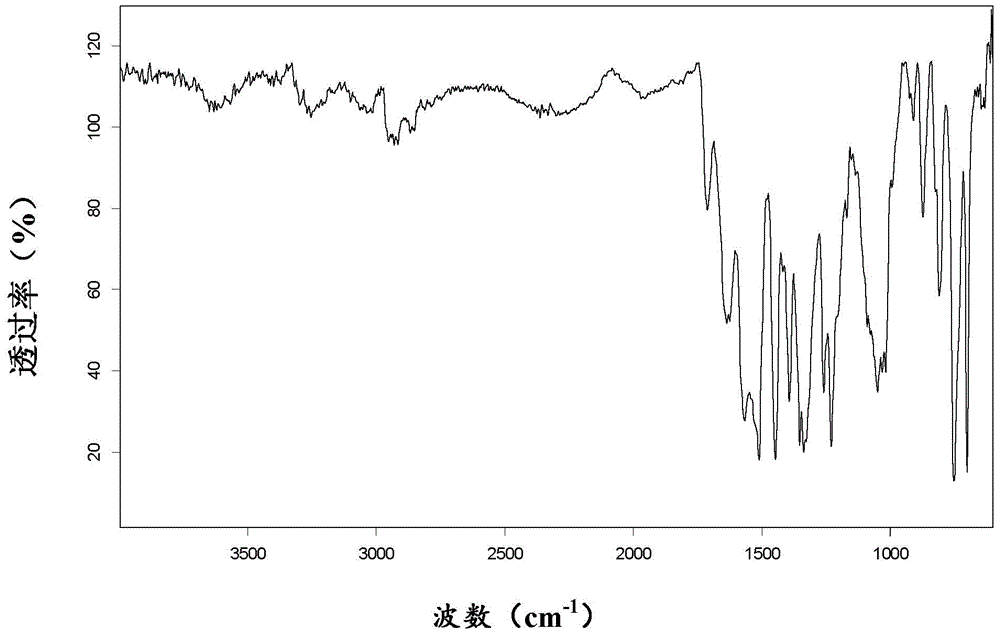
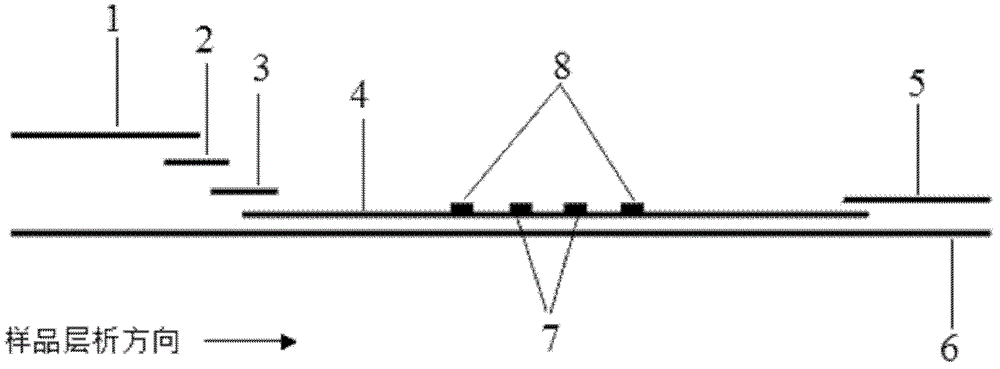
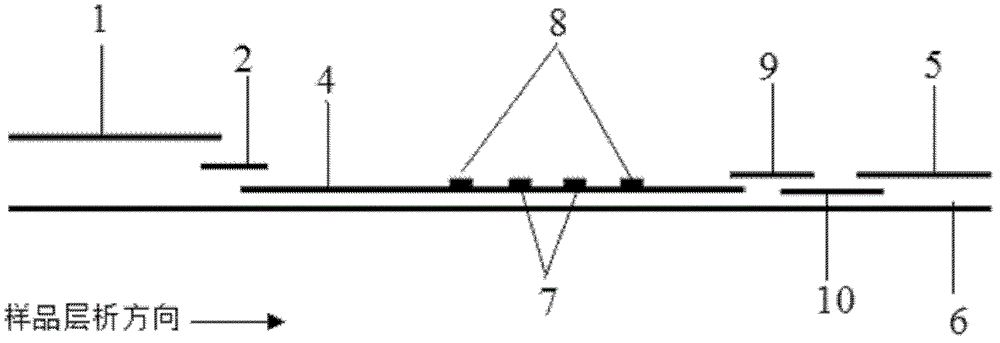
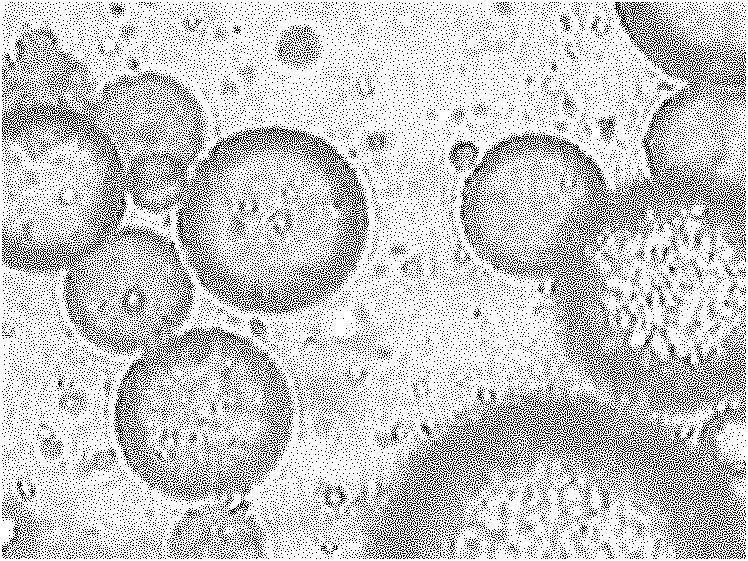Patents
Literature
48results about How to "Conducive to accurate quantification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
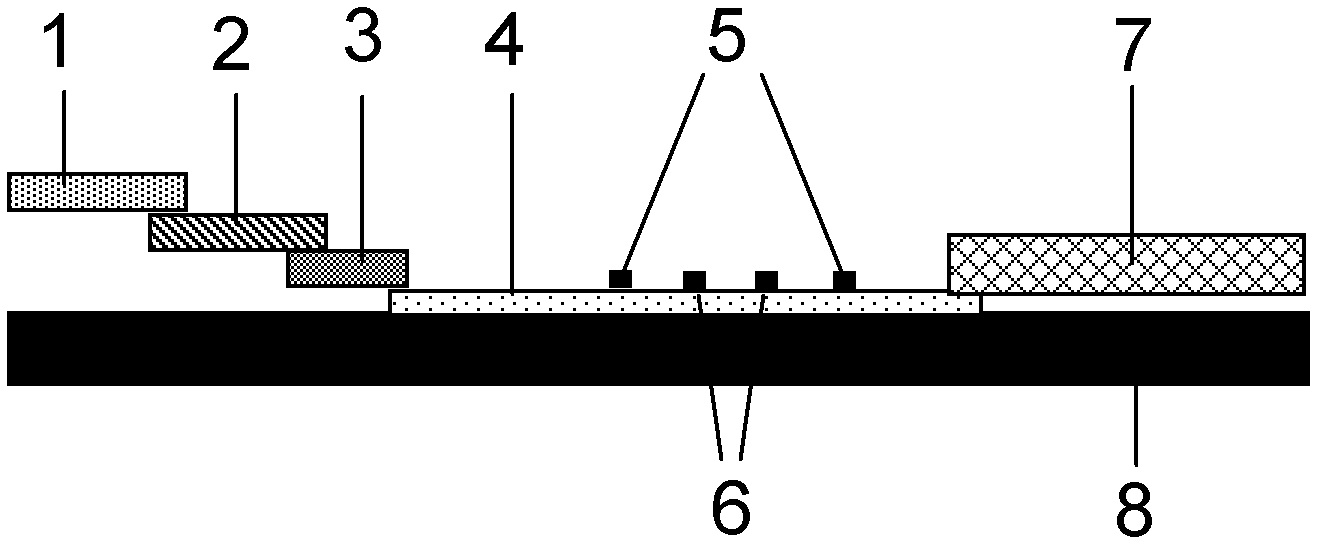
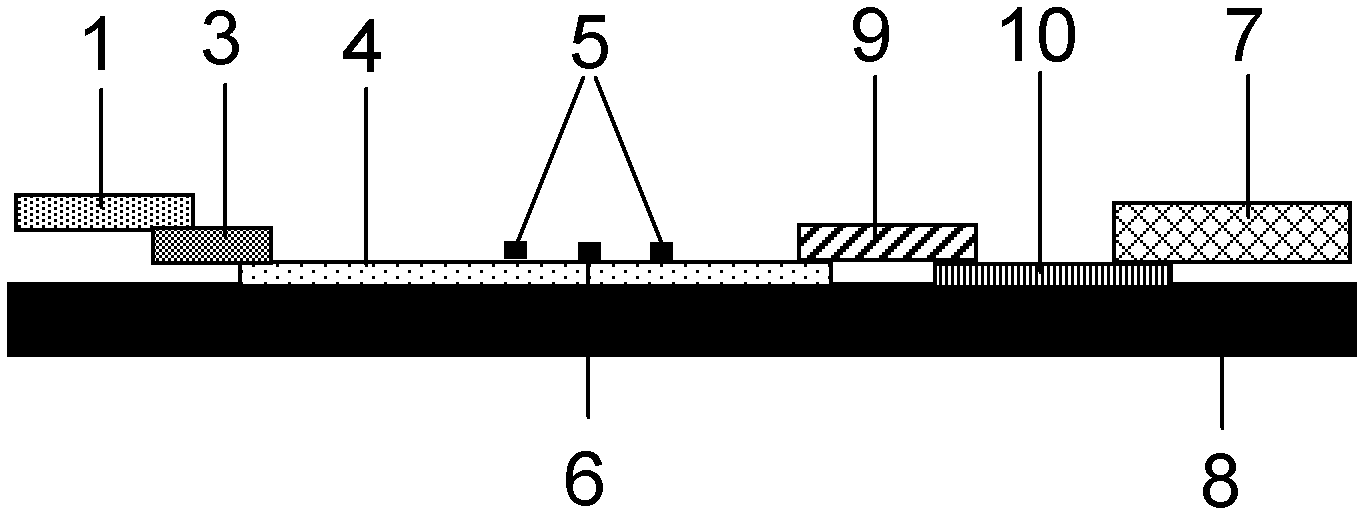
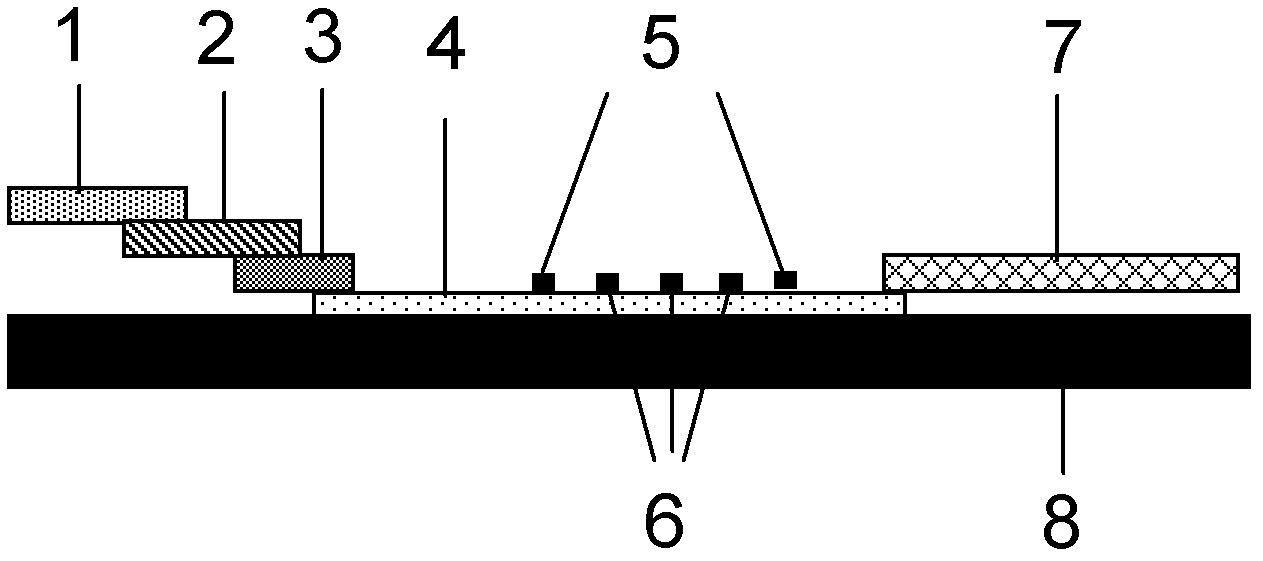
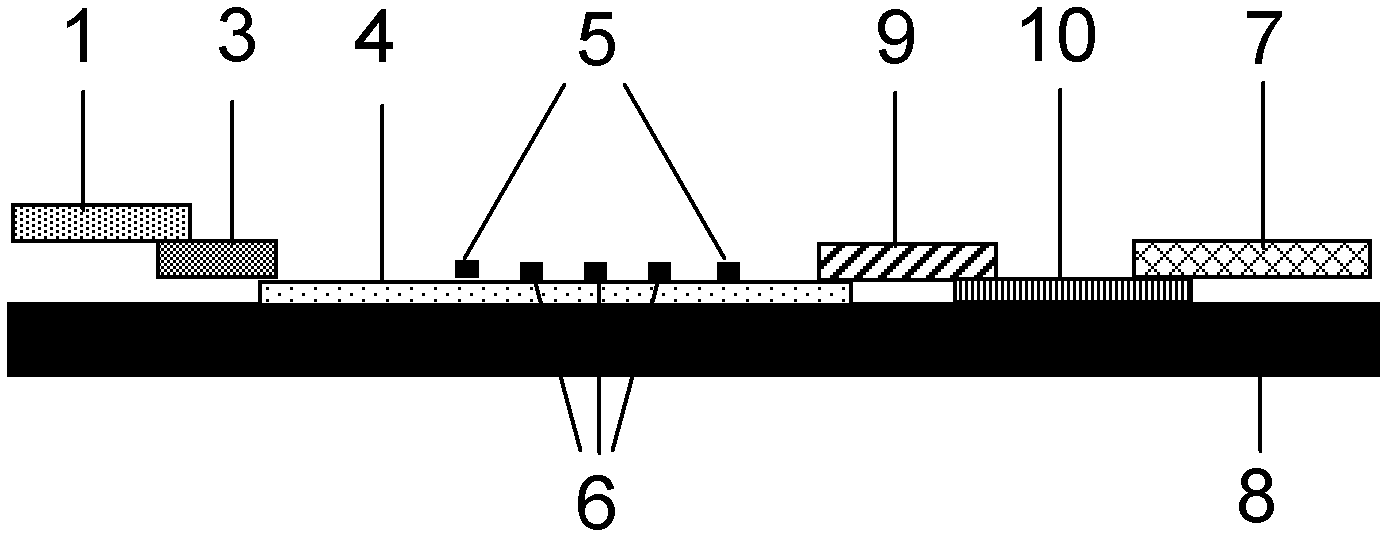
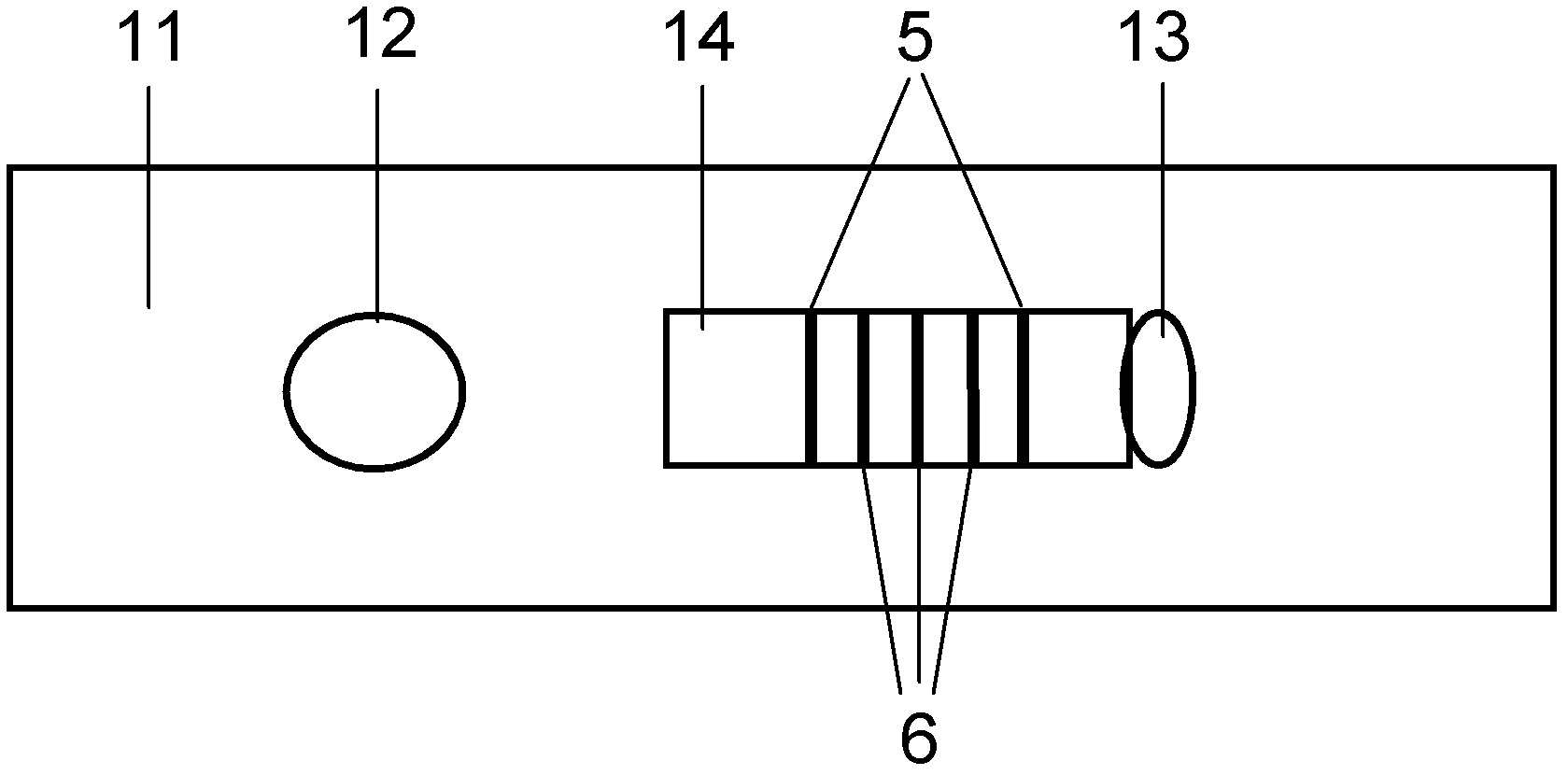
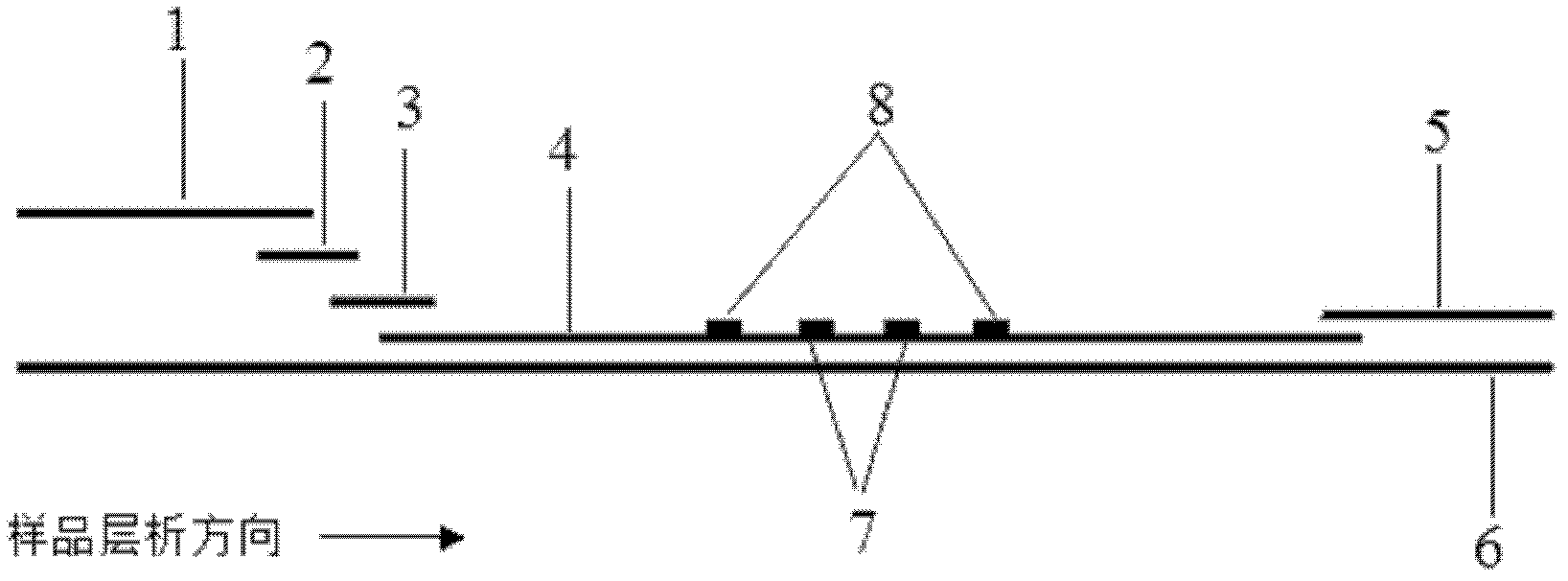
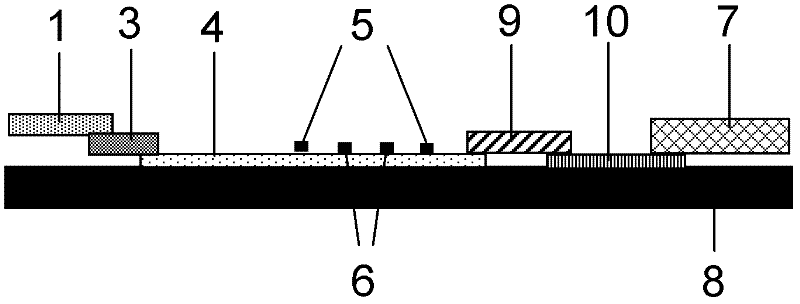
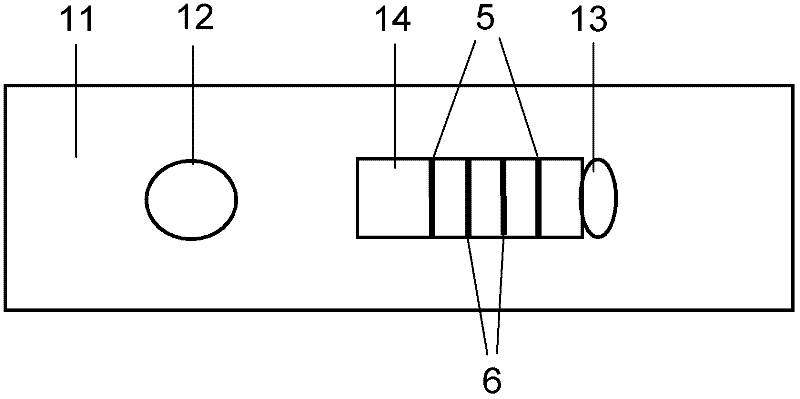
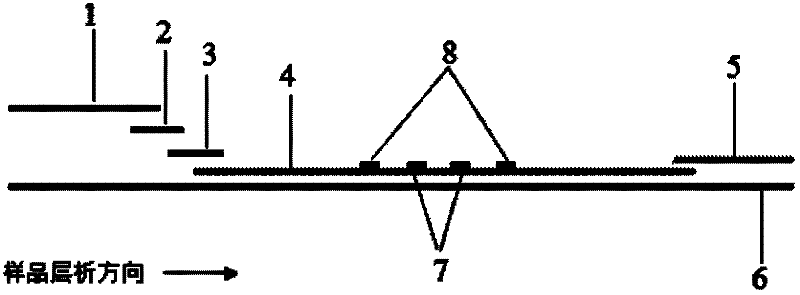
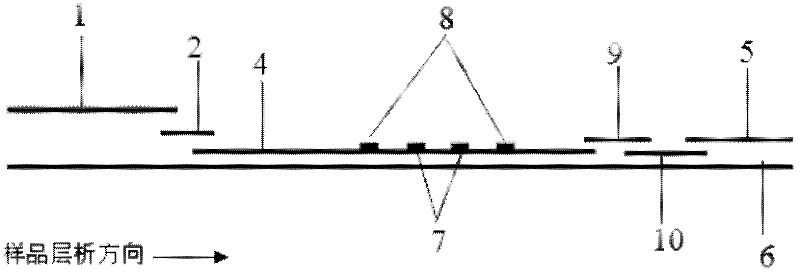
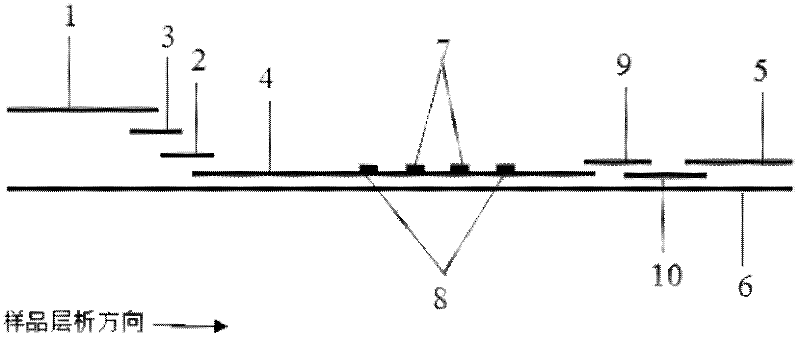
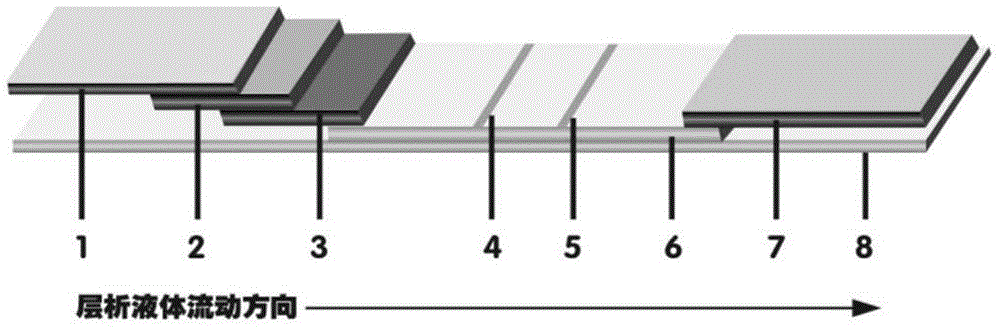
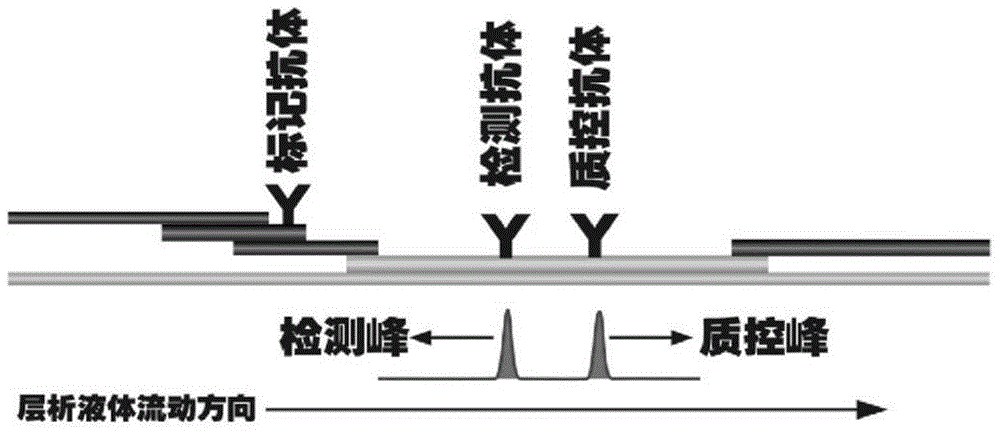
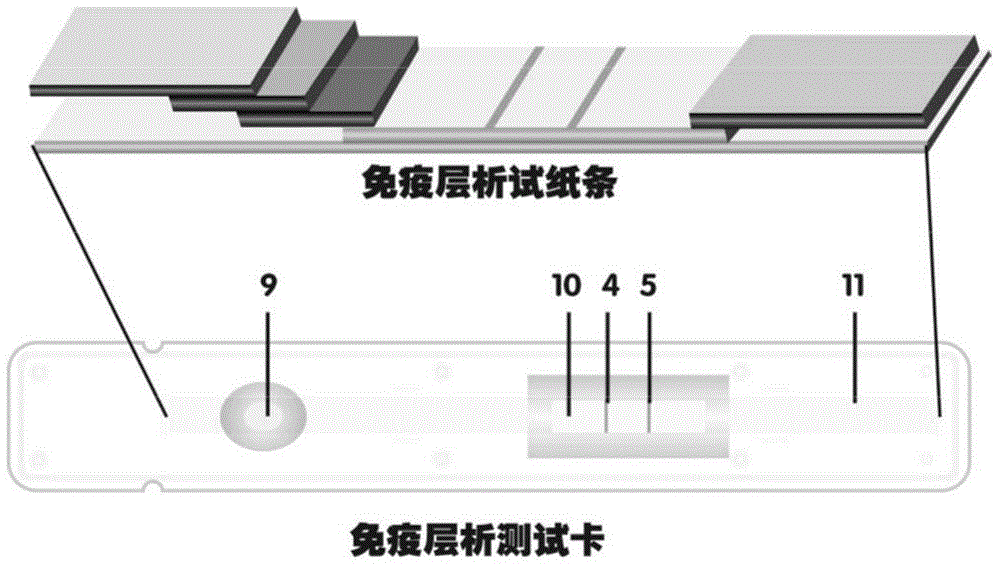
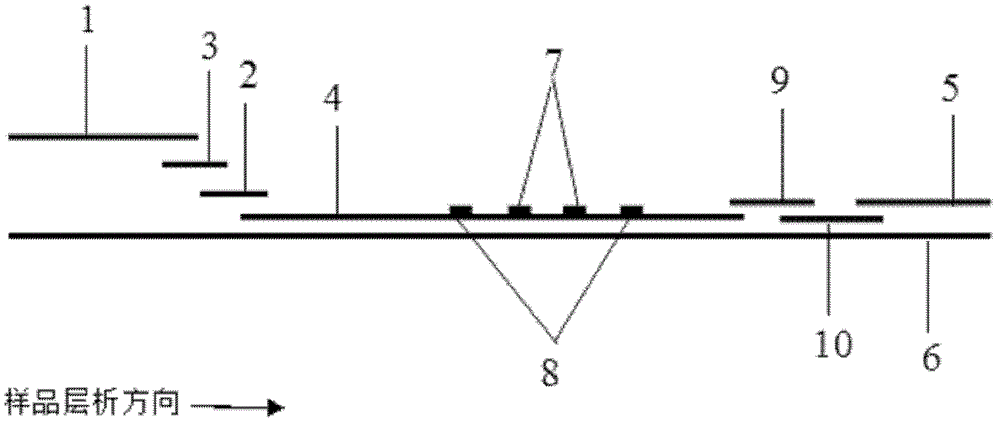
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
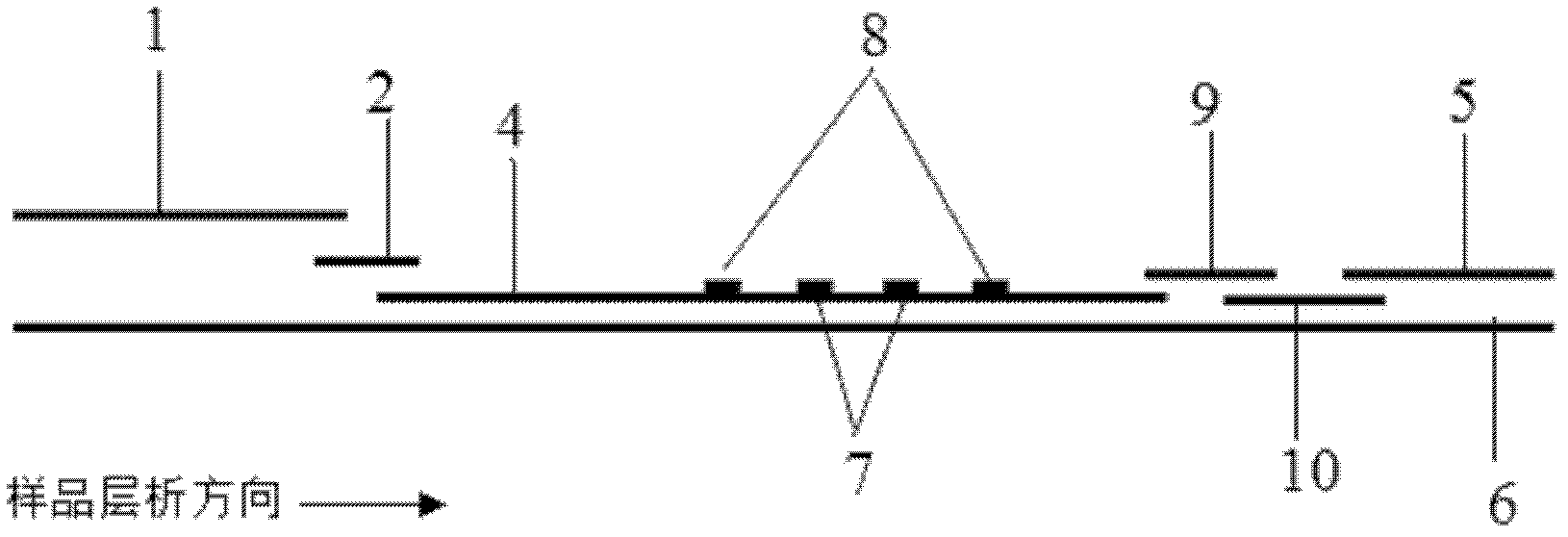
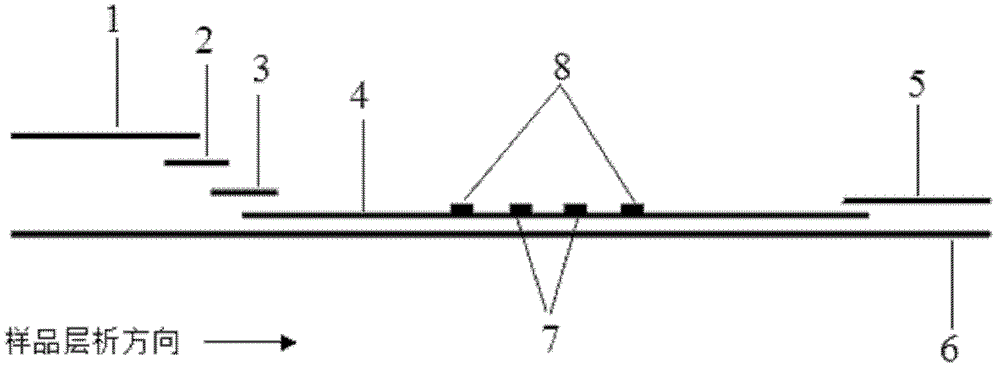
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
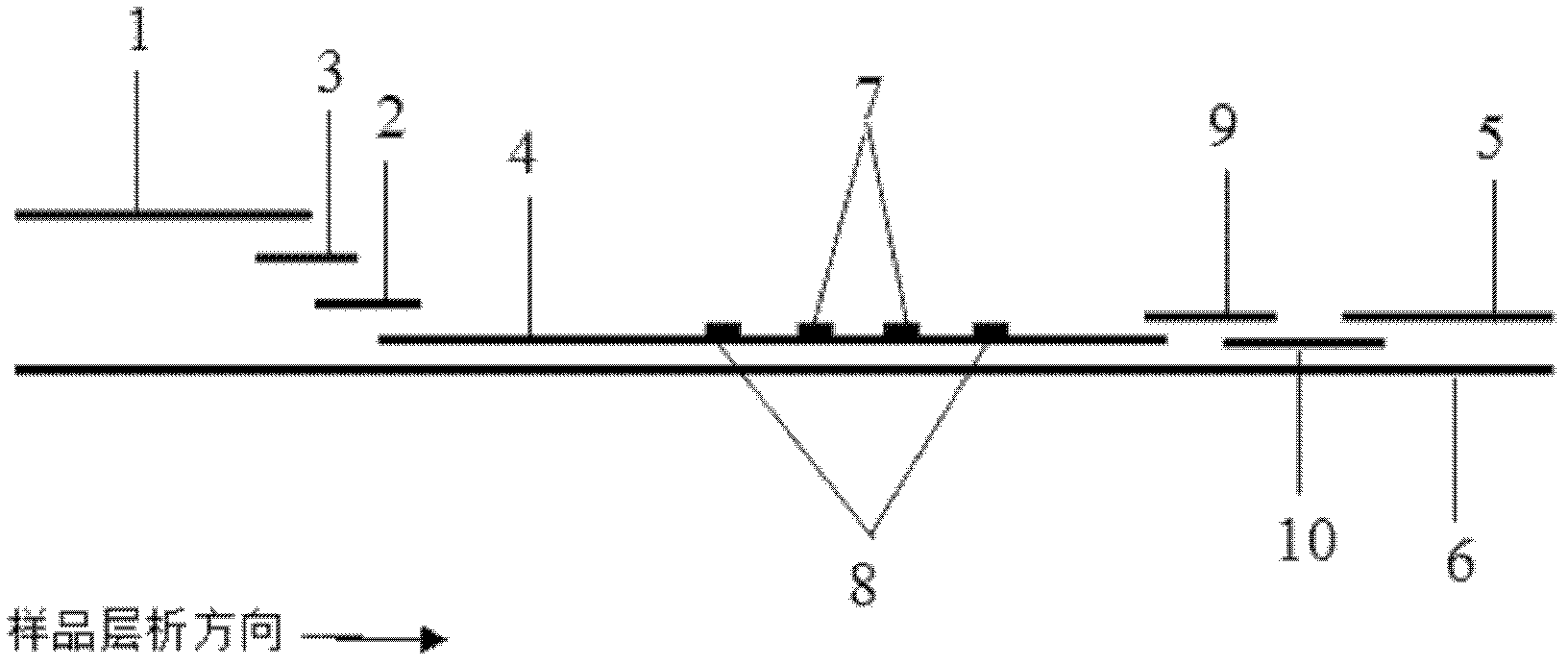
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
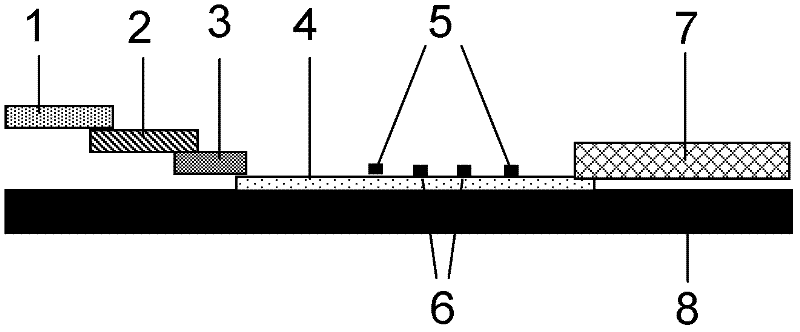
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
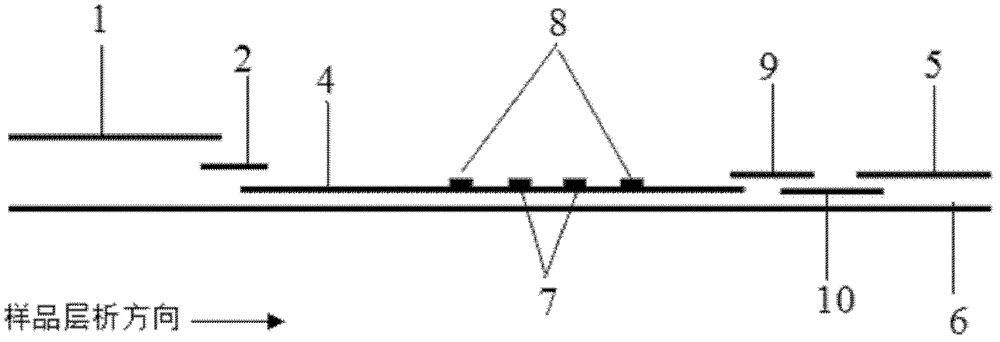
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
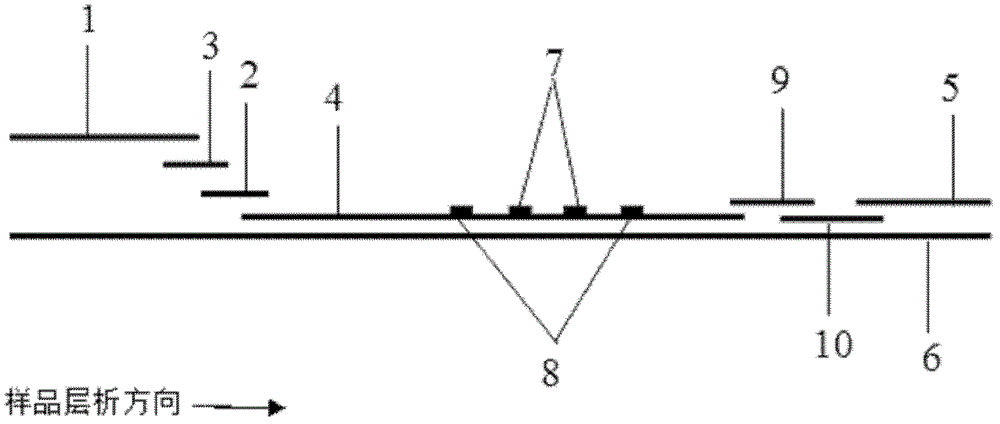
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Manufacture method for composite-material cementing structural test piece with defect of air holes in glue layer
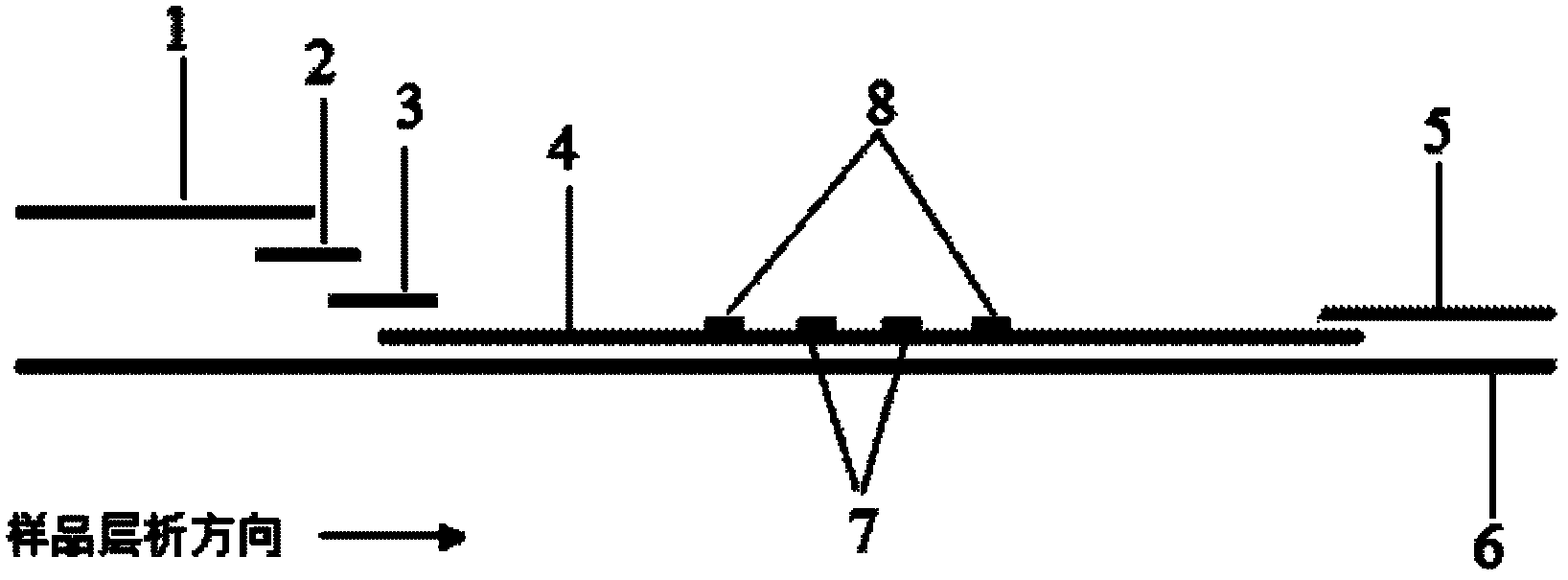
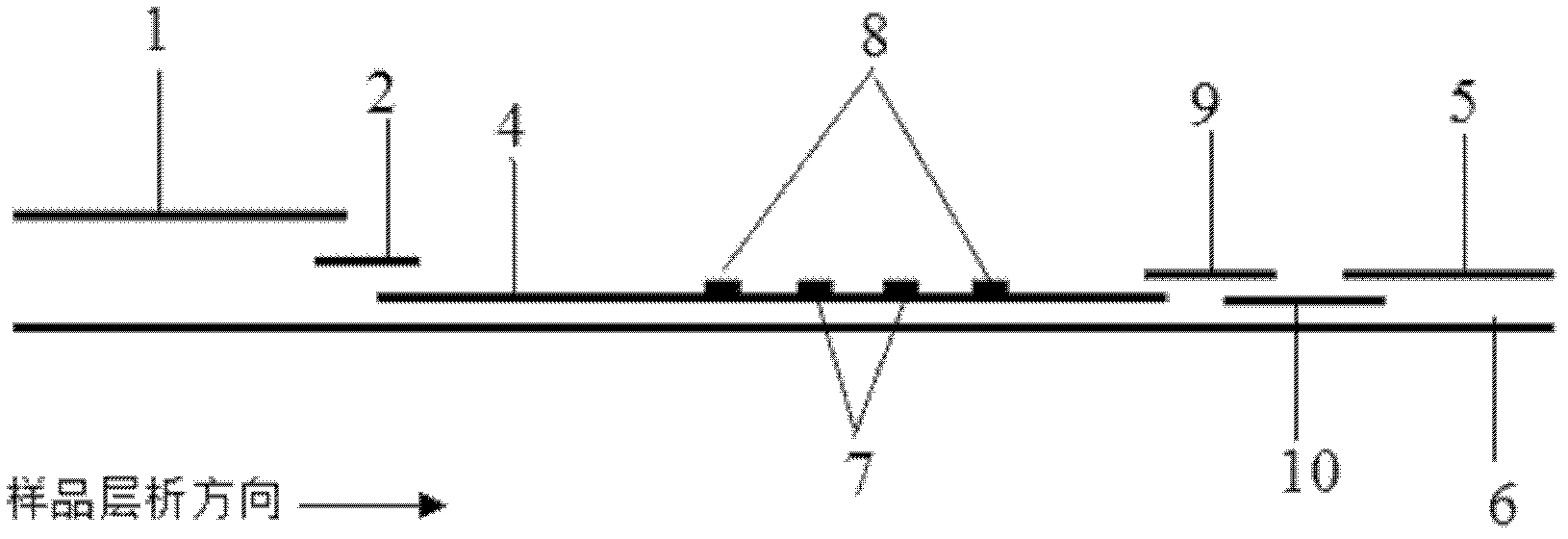
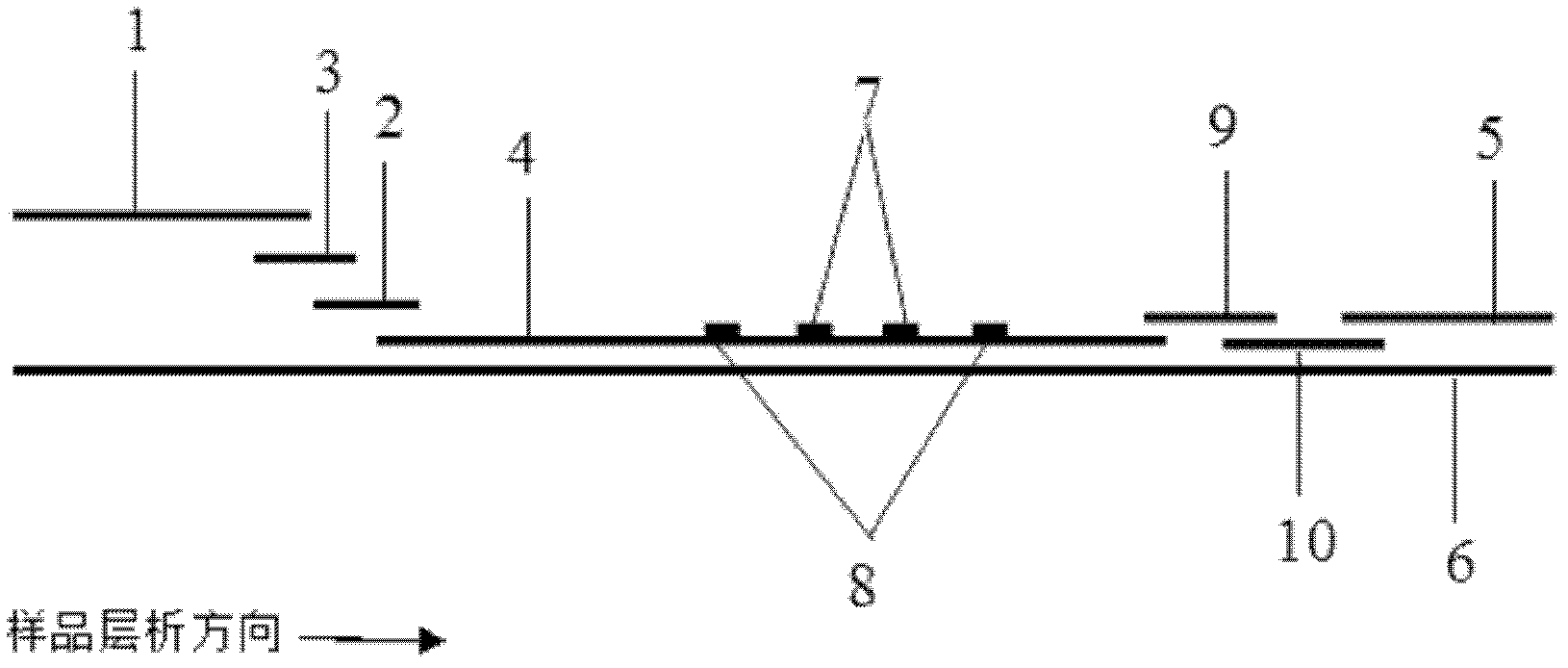
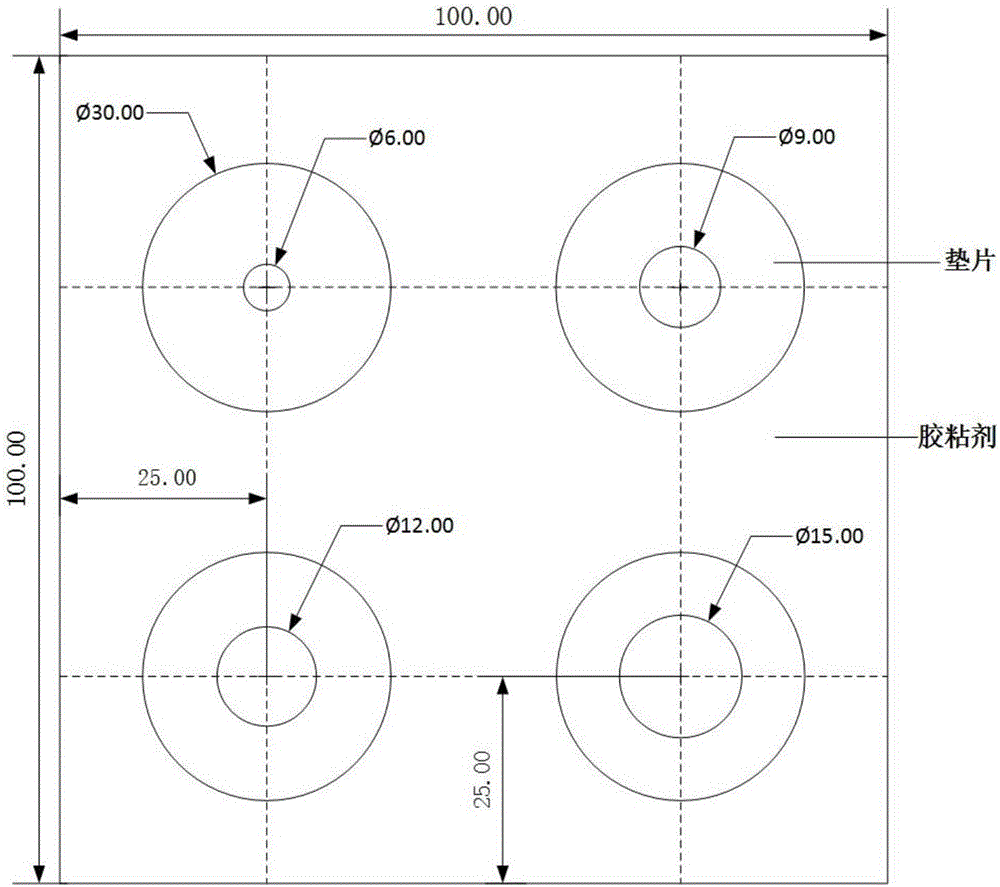
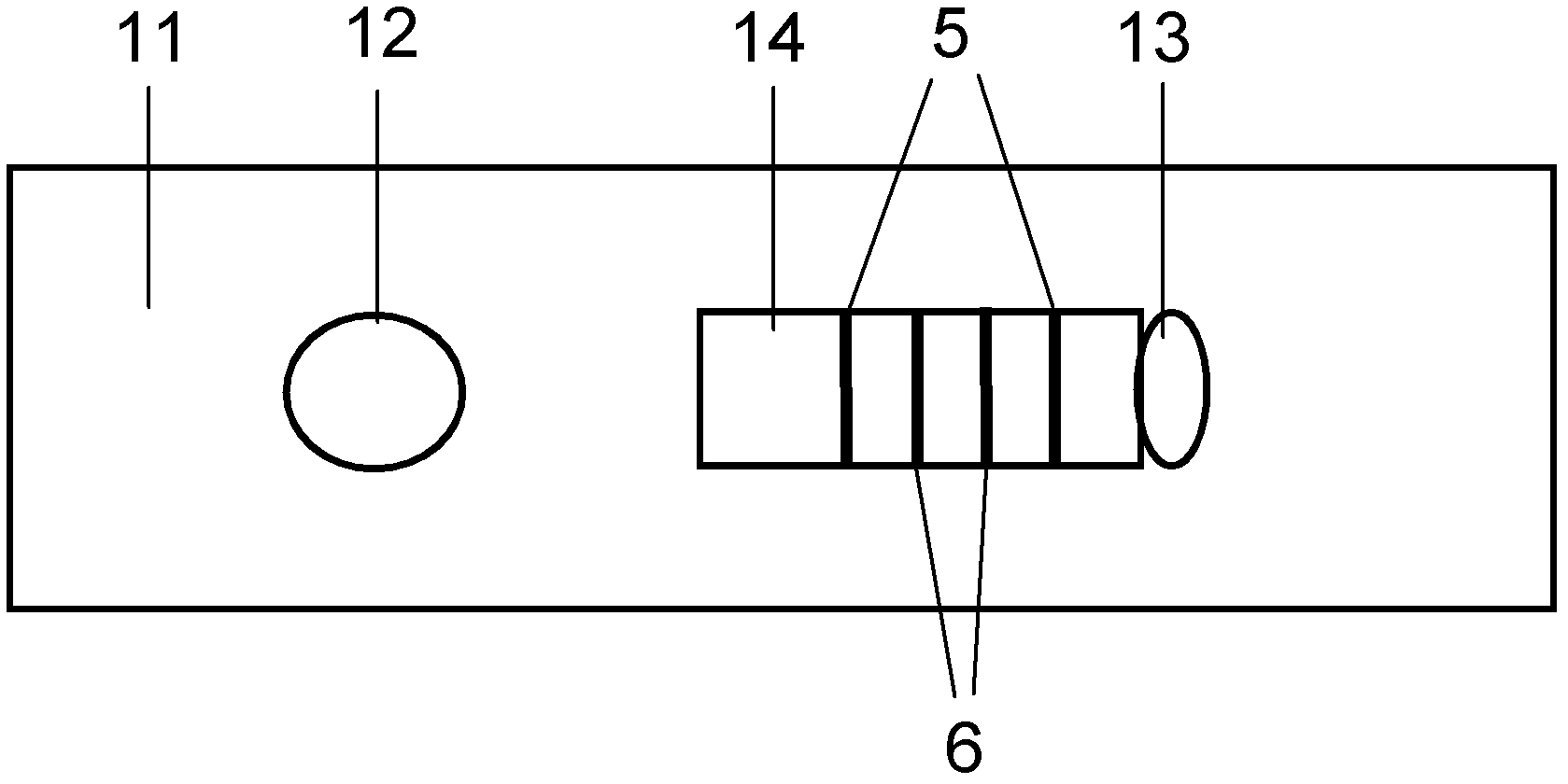
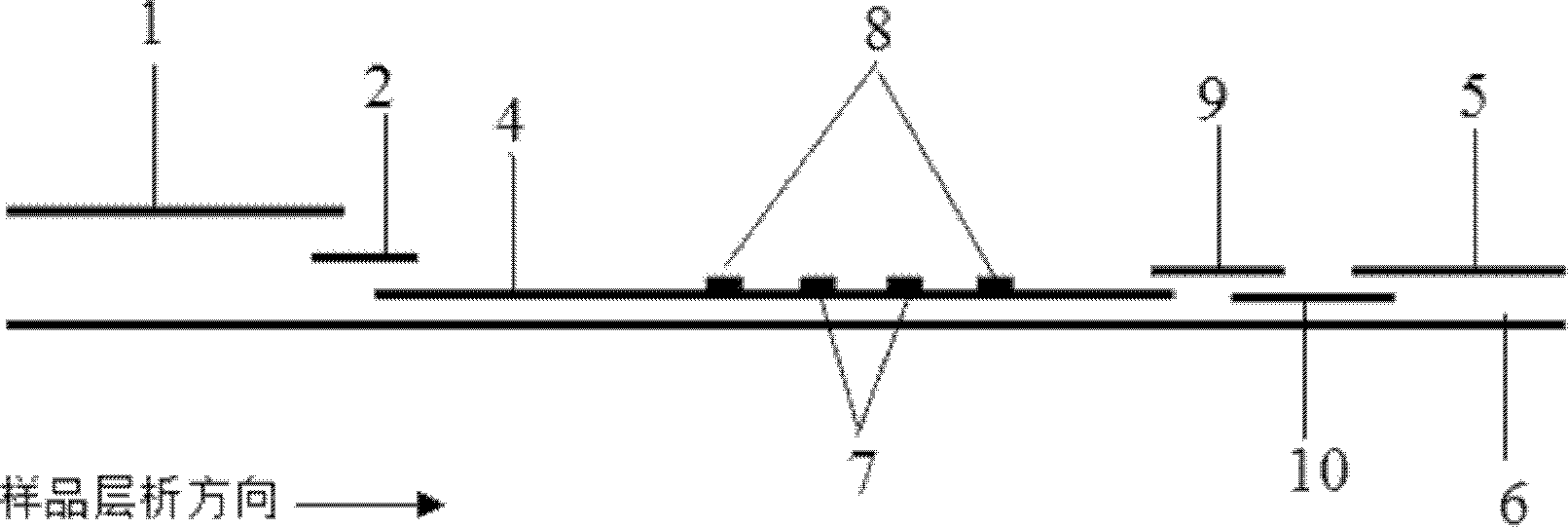
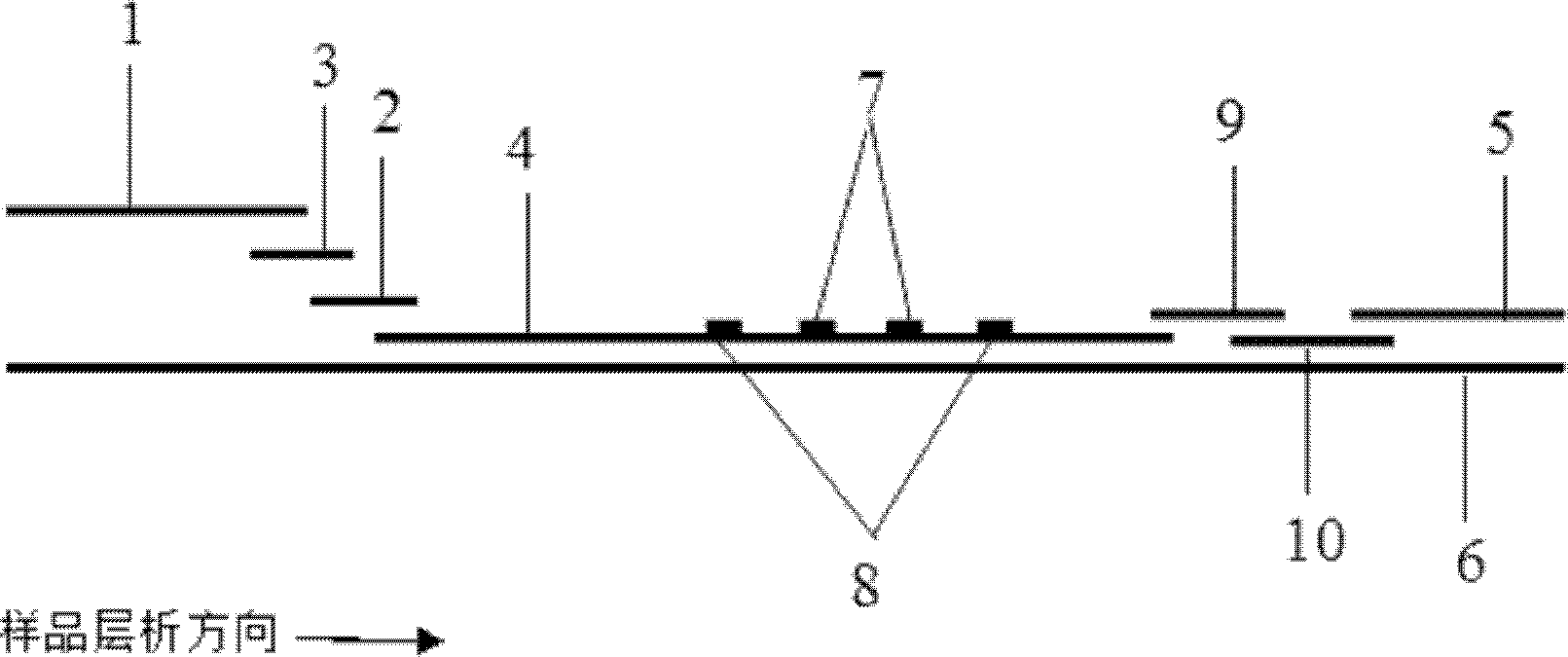
ActiveCN106093211AConducive to accurate quantificationClear identificationMaterial analysis using sonic/ultrasonic/infrasonic wavesPreparing sample for investigationNon destructiveNondestructive testing
The invention provides a manufacture method for a composite-material cementing structural test piece with a defect of air holes in a glue layer, and belongs to non-destructive detection field. The method realizes simulation of the air hole defect in the glue layer of the composite-material multi-layer cementing structure by employing 3D printing technology for preparing a glue-layer pad, and solves the technical problems that the size, shape, thickness and the like of an air hole defect in the glue layer cannot be quantitatively designed by employing the prior art. The method possesses the advantages of real and reliable simulation, high quantization precision, good expandability and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173ASolve the backgroundSolve the signal indistinguishableMaterial analysisDiseaseCreatine kinase
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of acute myocardial infarction marker-creatine kinase isoenzyme (CK-MB). The fluorescence immunochromatographic assay for quantitative detection of the CK-MB realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the CK-MB, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Analysis method applied to measuring residual quantity of sulfonylurea herbicide in plants
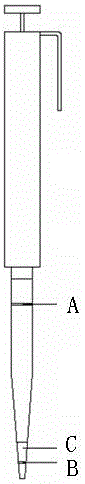
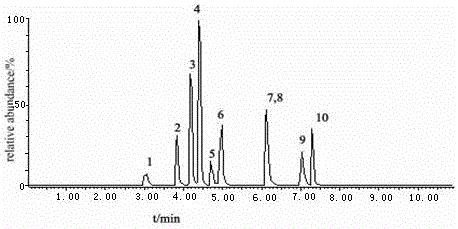
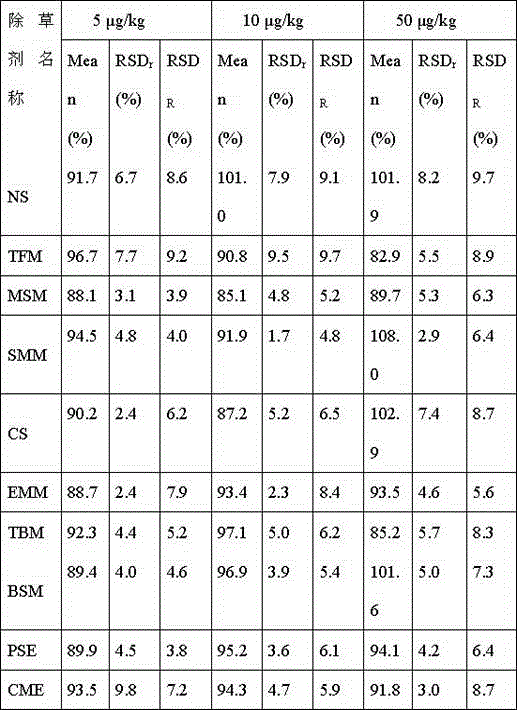
The invention discloses an analysis method applied to measuring residual quantity of sulfonylurea herbicide in plants. The method comprises the first step of preparing a stock solution, the second step of preparing extraction head through a QuEChERS method, the third step of processing samples and the fourth step of setting detection conditions of UPLC-MS / MS and conducting sample introduction. The method has the advantages that pretreatment is conducted through an extraction device, the sample purifying process is simple and quick, samples can be obtained through simple absorbing-pushing, results are accurate, and detection is quick; secondly, a sieve in the extraction head allows dispersion extraction, adsorbent, salt and other particulate matter can be removed out of the solution, the number of impurities is reduced, and convenience is brought to accurate quantitation; quantitation is accurate, sensitivity is high, operation is easy and quick, the consumption of the solvent is small, and the requirement for pesticide residue analysis can be met; the improved QuEChERS-method device is used for measuring the residue quantity of 10 kinds of sulfonylurea herbicide in plants.
Owner:NANJING INST OF ENVIRONMENTAL SCI MINIST OF ECOLOGY & ENVIRONMENT OF THE PEOPLES REPUBLIC OF CHINA
Quantitative lime slurry mixing device for flue gas desulfurization
PendingCN112957984AEasy to collectQuantitative preventionGas treatmentTransportation and packagingProcess engineeringSlurry
The invention discloses a quantitative lime slurry mixing device for flue gas desulfurization. The quantitative lime slurry mixing device comprises a workbench, a discharging mechanism is arranged on one side of the workbench, a quantitative feeding mechanism is arranged on the workbench, and a stirring mechanism is arranged on one side of the quantitative feeding mechanism. The device has the beneficial effects that the device can be used for quantifying, feeding, stirring, discharging and the like at the same time, and the working efficiency is improved.
Owner:EQUIP MFG FACTORY HEBEI HONGDA ENVIRONMENT ENG CO LTD
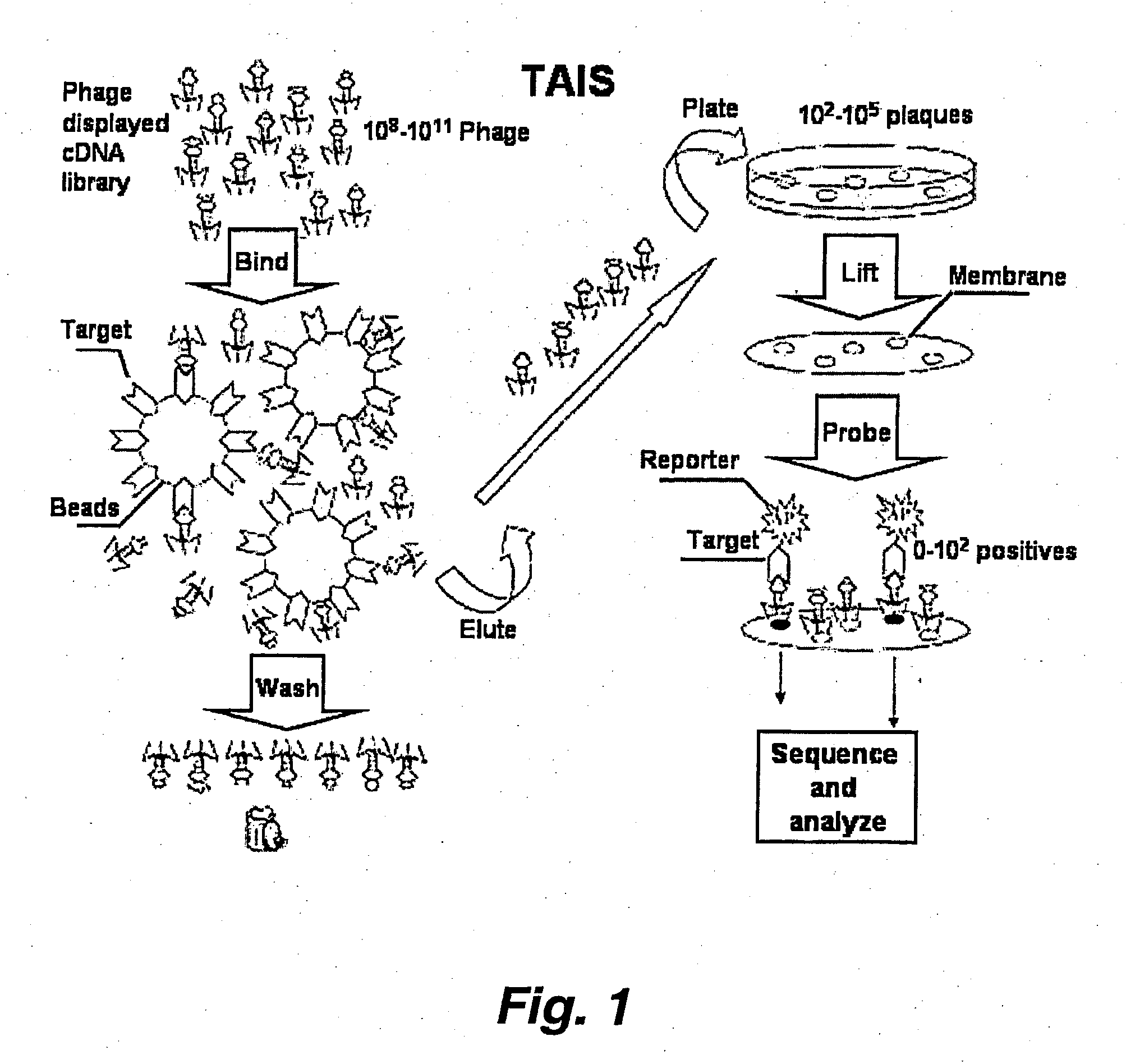
Targeted-assisted iterative screening (tais):a novel screening format for large molecular repertoires
InactiveUS20060099713A1Improve efficiencyHighly simpleMicroorganism librariesBiological testingCDNA libraryNEDD4
This invention provides a new in vitro screening method for the detection of protein-protein and other interactions. The method has been developed and applied to a commercial cDNA library to search for novel protein-protein interactions. PDZ, WW and SH3 domains from PSD95, Nedd4, Src, Abl and Crk proteins were used as test targets. 12 novel putative and 2 previously reported interactions were identified for 6 protein interaction modules in test screens. The novel screening format, dubbed TAIS (target-assisted iterative screening), provides an alternative platform to existing technologies for a pair-wise characterization of protein-protein, and other, interactions.
Owner:THE BUCK INST FOR RES ON AGING
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
Oxdiazole compound solvate and preparation method thereof
ActiveCN105461650AFavorable viscosityImprove bioavailabilityOrganic active ingredientsSenses disorderBenzoic acidPharmaceutical drug
The present invention relates to the dimethyl sulfoxide solvate of 3-[5-(2-fluorophenyl)-[1,2,4]oxdiazole-3-yl]benzoic acid, wherein the dimethyl sulfoxide solvate has characteristics of good stability, high purity, excellent particle and excellent shape, and is suitable for pharmaceutical preparation applications. The present invention further relates to a preparation method of the dimethyl sulfoxide solvate, a pharmaceutical composition of the dimethyl sulfoxide solvate, and uses of the dimethyl sulfoxide solvate in preparation of drugs for treatment of genetic diseases.
Owner:SOLIPHARMA
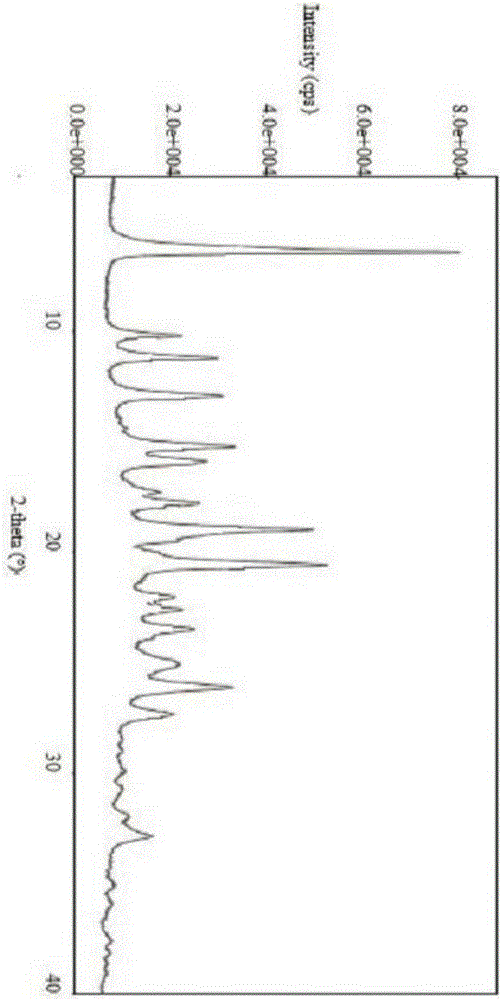
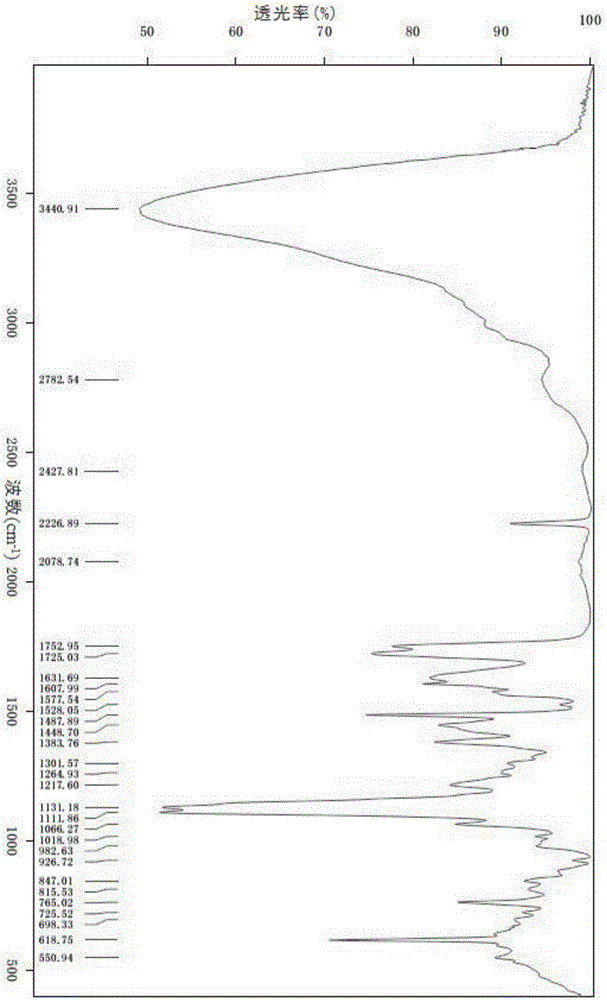
Isavuconazole sulfate crystal and preparation method thereof
ActiveCN106565699ACrystal stableHigh purityOrganic chemistry methodsPharmaceutical delivery mechanismSulfateRe crystallization
The invention relates to an isavuconazole sulfate crystal form, and a preparation method and a medicinal composition thereof. The crystal form has the advantages of good stability, high purity, good reappearance and suitableness for preparing medicinal preparations. The preparation method comprises the following steps: dissociating isavuconazole hydrochloride by using an alkali, carrying out salt exchange by using concentrated sulfuric acid and hydrogen peroxide, and carrying out re-crystallization. The method has the advantages of mild reaction conditions, simple post-treatment and high purity of the above product, and meets basic requirements of industrial amplified production.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
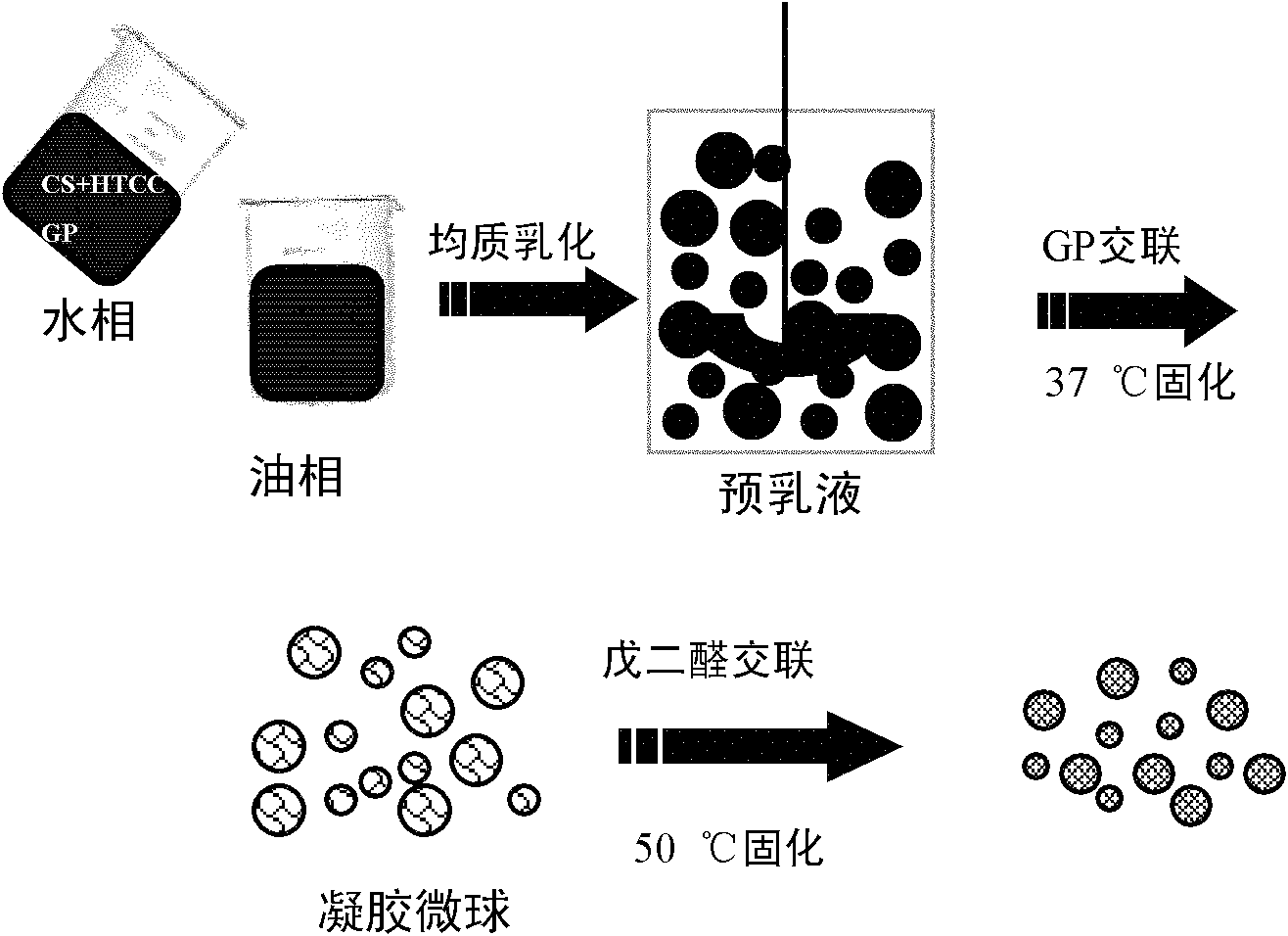
Gastric targeted drug carrier and preparation method thereof
InactiveCN102652833AReduce embedding rateHigh embedding ratePharmaceutical non-active ingredientsEmbedding rateMicrosphere
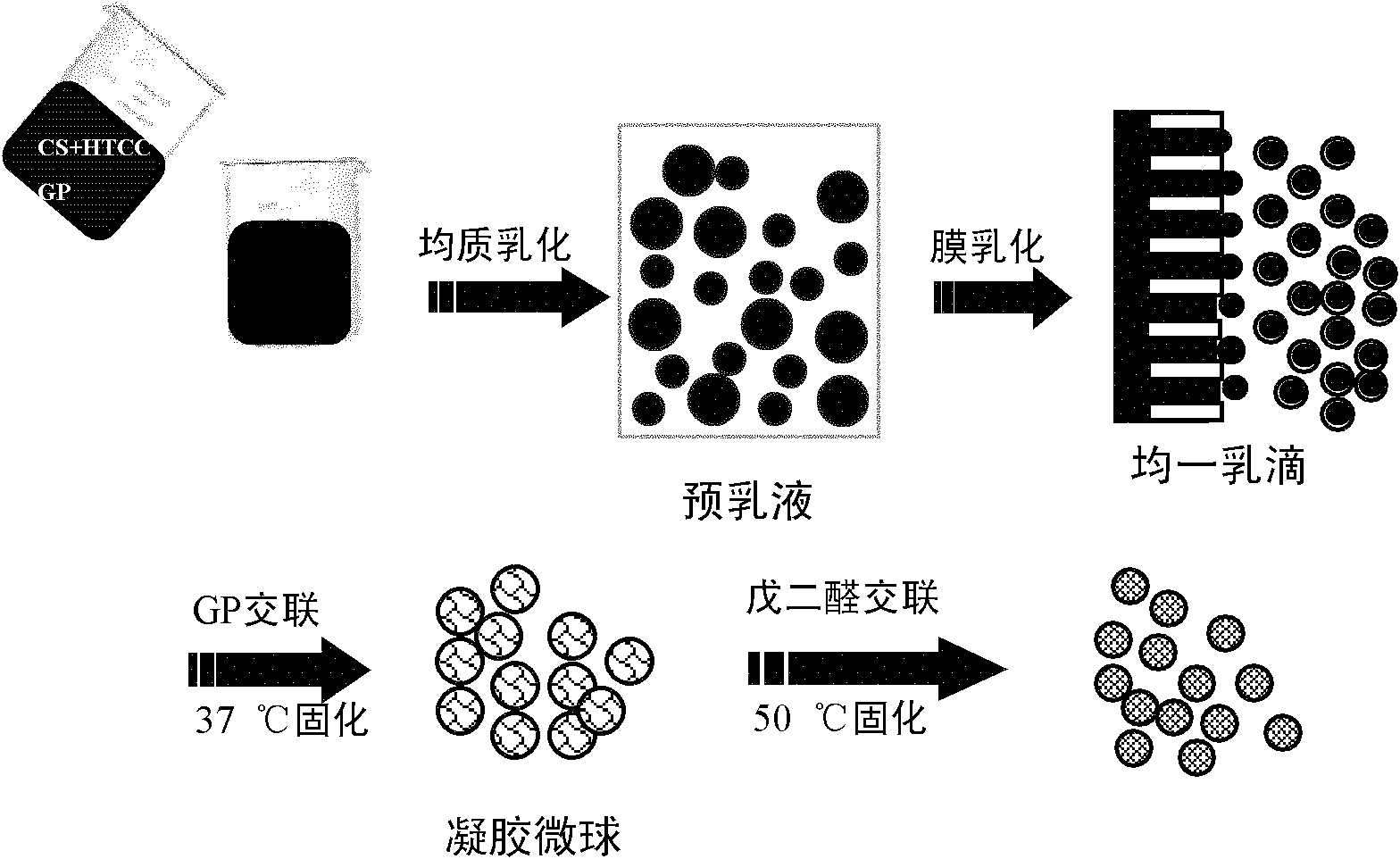
The invention relates to the field of drug carrier preparation, in particular to a gastric targeted drug carrier and a preparation method thereof. The gastric targeted drug carrier is prepared from the following raw materials: drug-containing emulsion and a crosslinking agent, wherein the drug-containing emulsion contains chitosan, chitosan quaternary ammonium salt and sodium glycerophosphate; and the crosslinking agent is used for crosslinking the emulsion into microspheres. The preparation method comprises the following steps: 1) preparing the drug-containing emulsion: with a aqueous solution of chitosan, chitosan quaternary ammonium salt and sodium glycerophosphate as an aqueous phase W, preparing the drug-containing emulsion with the aqueous phase, drugs and an oil phase containing anemulsifying agent; and 2) heating and stirring the drug-containing emulsion prepared in the step 1), crosslinking the chitosan quaternary ammonium salt and sodium glycerophosphate contained in the drug-containing emulsion to obtain gel microsphere suspension, heating the suspension and adding the crosslinking agent to carry out secondary crosslinking to solidify the gel microspheres. The drug carrier has the advantages of uniform and controllable grain size, high embedding rate and good dispersibility and simultaneously meets the requirements of the gastric targeted drug delivery carrier.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for testing content of corrosive sulphur in insulating oil
ActiveCN103954521AEasy to operateConducive to accurate quantificationMaterial weighingResonance oscillationTemperature control
The invention discloses a method for testing content of corrosive sulphur in insulating oil. The method comprises the following steps: (1) taking an insulating oil sample used by an authorized transformer; (2) coating a quartz crystal resonance oscillation piece by using the insulating oil sample; (3) introducing nitrogen; (4) recording the frequency f1 of the quartz crystal resonance oscillation piece; (5) controlling the temperature to be between 90-150 DEG C and keeping for 0.5-10 hours; (6) recording the frequency f2 of the quartz crystal resonance oscillation piece; (7) calculating the frequency change delta f of the quartz crystal resonance oscillation piece; and (8) calculating the content of corrosive sulphur in the insulating oil used by the authorized transformer. By using the method, the content of corrosive sulphur in the oil of the transformer can be conveniently, accurately and quantitatively tested, and therefore, the device is easy and convenient to operate, and has the important guiding significance on judgment of incipient faults of transformers and development of state maintenance work of the transformers.
Owner:STATE GRID CORP OF CHINA +2
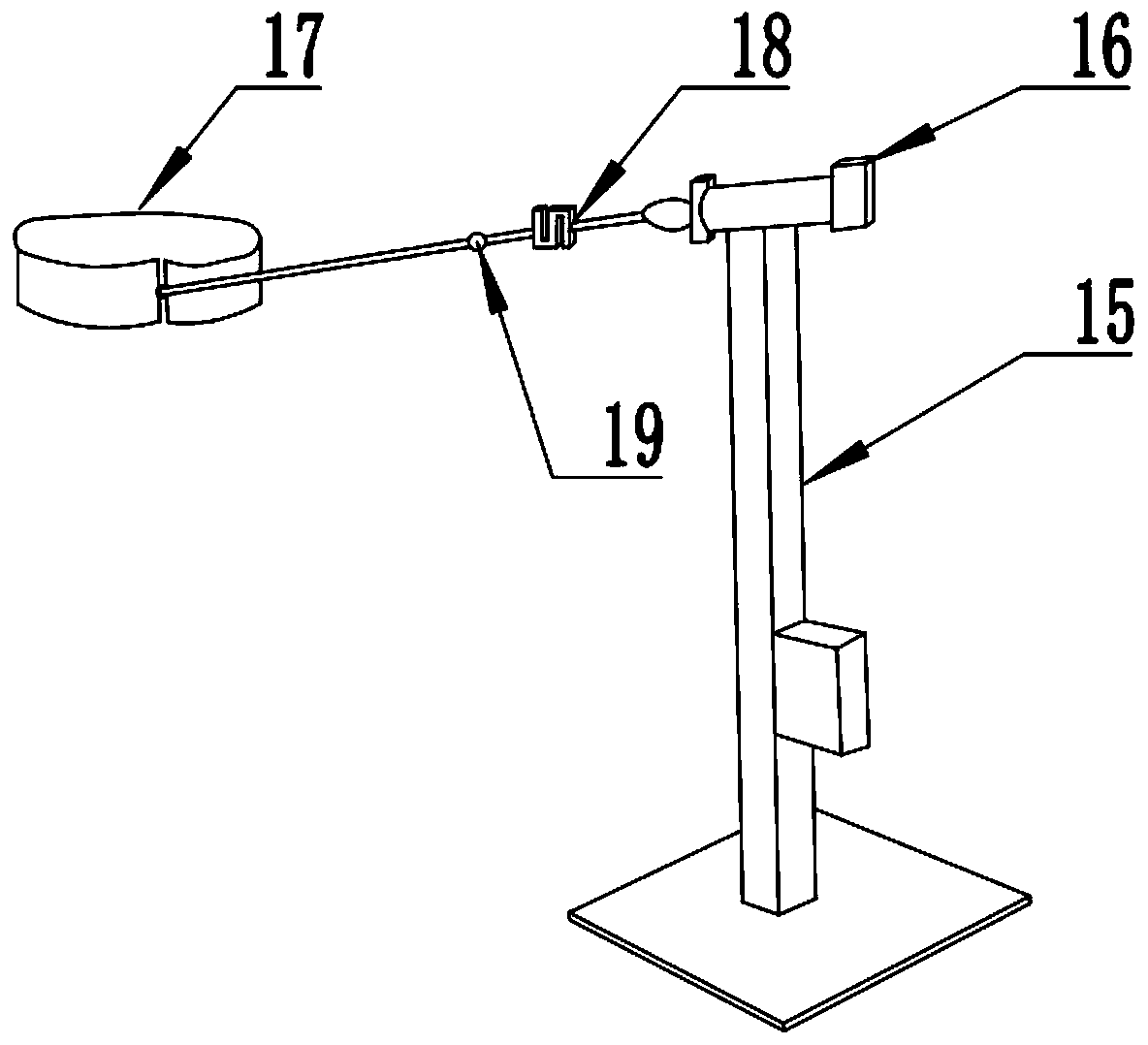
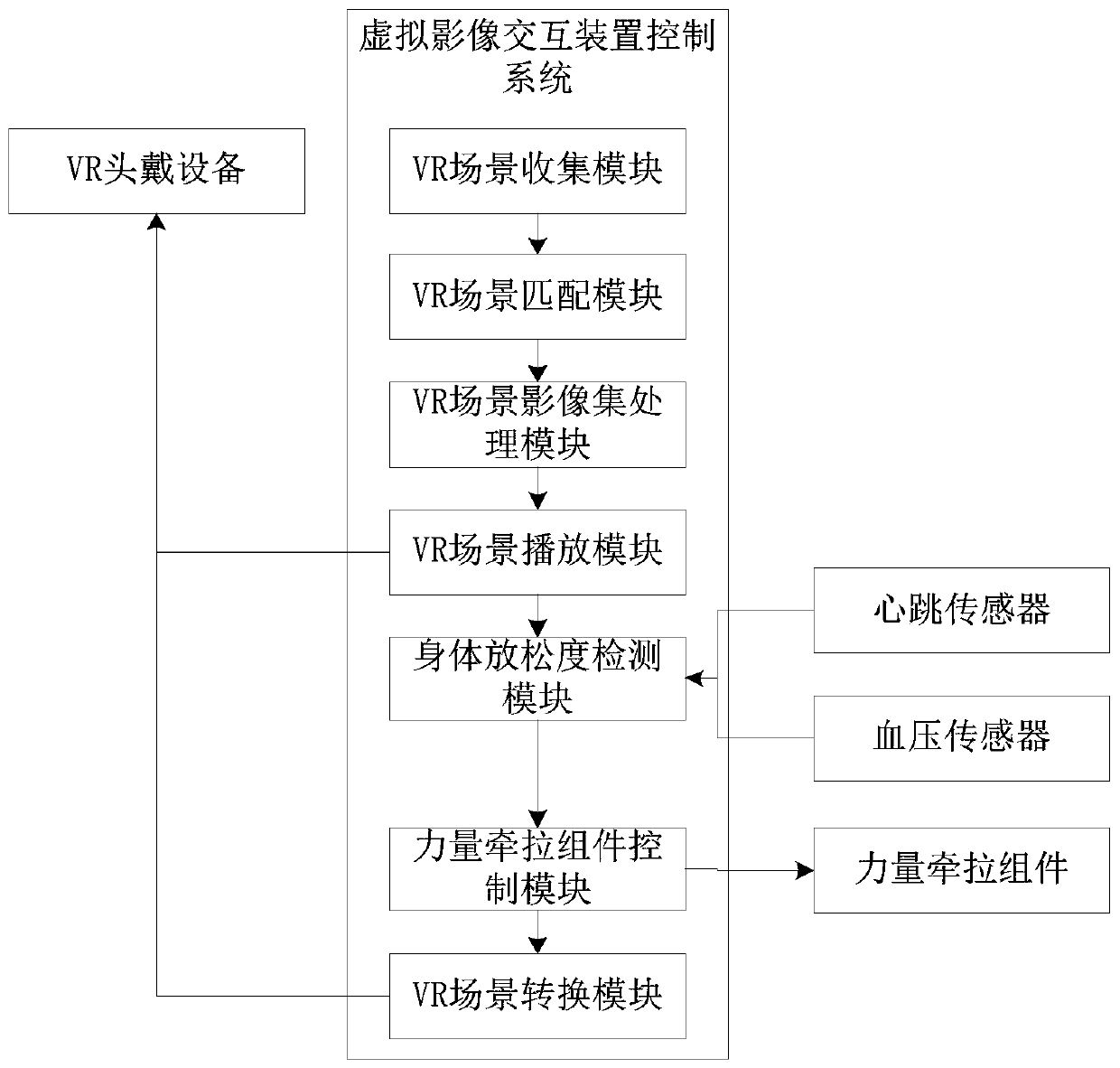
Trunk core muscle group balance control capability evaluation system
ActiveCN110448274AAvoid test errorImprove securityDiagnostic recording/measuringSensorsMuscle groupComputer module
The invention relates to the technical field of sports equipment, and particularly relates to a trunk core muscle group balance control capability evaluation system. The system comprises a sitting support, a strength traction assembly and a three-dimensional trunk dynamic analysis module. Wherein the sitting support is used for fixing the body trunk of a subject; the strength traction assembly isused for applying detection stress to the body trunk of the subject to generate oscillation data of the body trunk; the three-dimensional trunk dynamic analysis module is used for acquiring the oscillation data of the body trunk, analyzing the index parameters of the trunk core muscle group balance control capability of the subject according to the oscillation data and the disturbance data, and carrying out system evaluation according to the index parameters. According to the method, the problems that only single presentation of the current evaluation methods can be realized, and accurate quantification and system evaluation cannot be realized in an existing evaluation method are solved.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Special purification column for aflatoxin M group and application
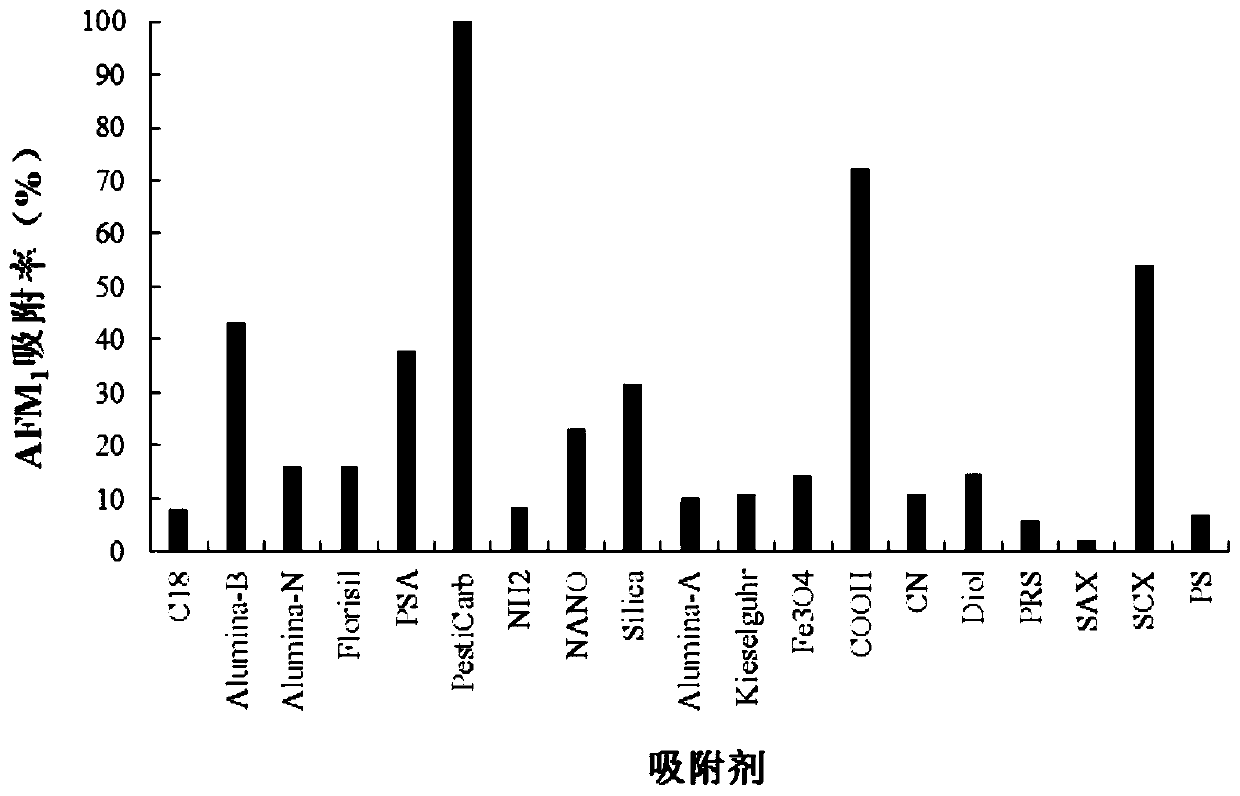
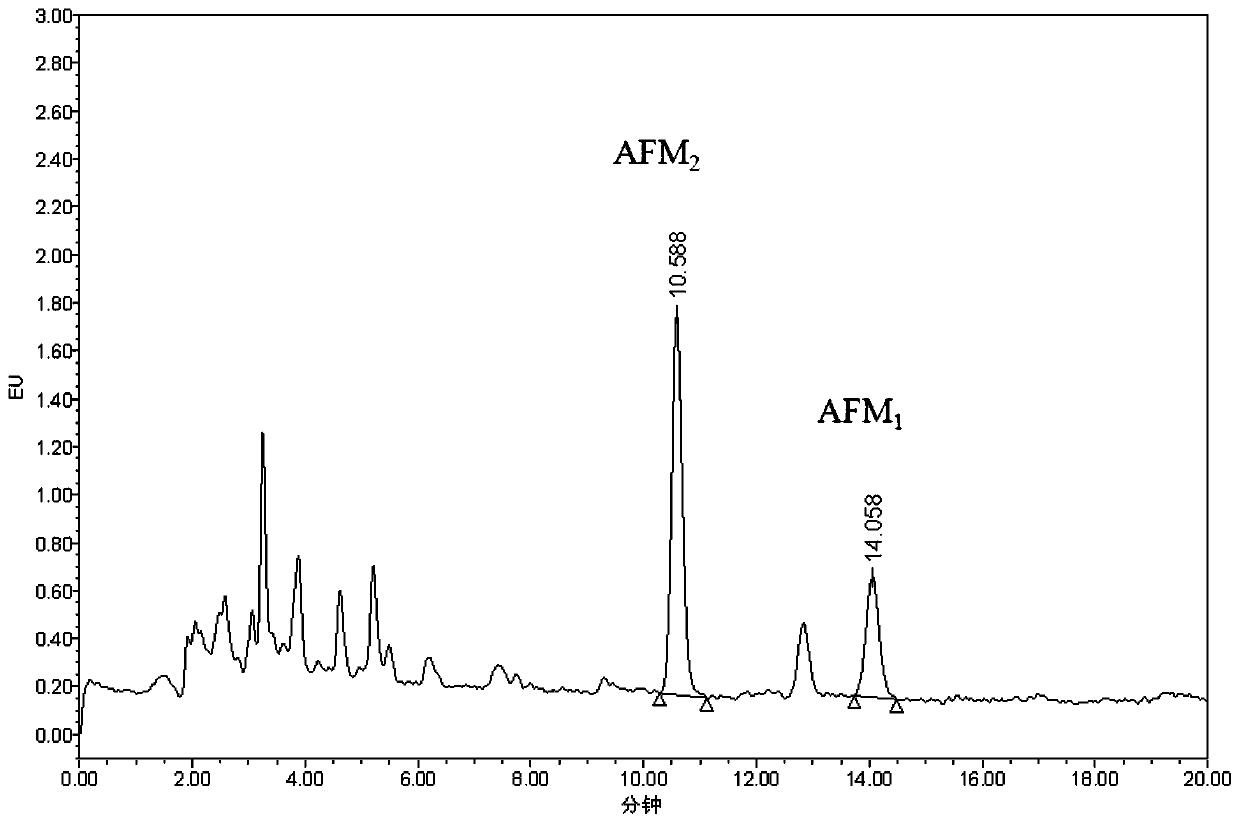
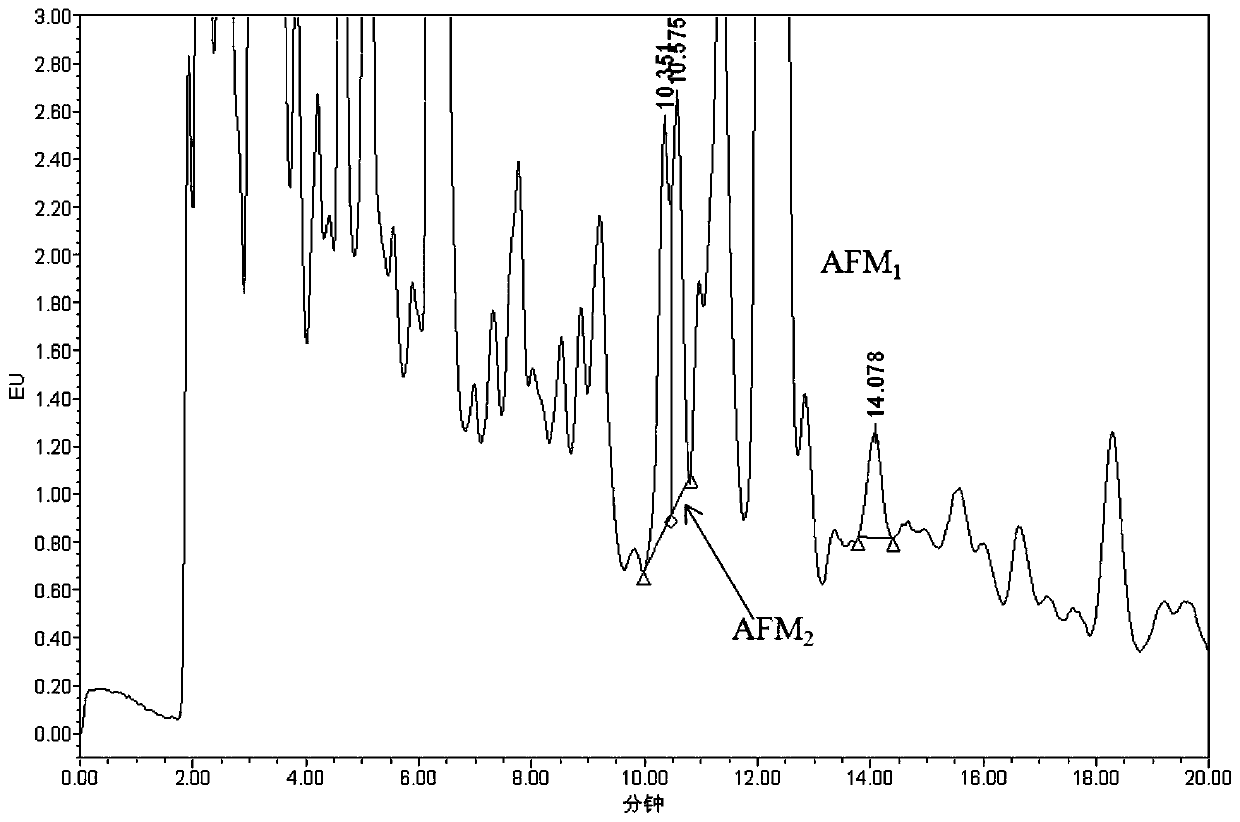
ActiveCN110646524ADoes not affect adsorption capacityImprove adsorption capacityComponent separationSolid sorbent liquid separationBiotechnologyAflatoxin M
The invention belongs to the field of milk and milk product quality safety detection, and particularly discloses a special purification column for an aflatoxin M group and an application. The specialpurification column comprises a column pipe; a lower sieve plate, a filler and an upper sieve plate are sequentially arranged in the column pipe from the liquid outlet end of the column pipe to the liquid inlet end of the column pipe; and the filler is a mixture of graphitized carbon and nano ferroferric oxide. The invention also discloses an application of the special purification column to the purification and detection of the aflatoxin M group in milk and milk products. The special purification column for the aflatoxin M group is simple and convenient to manufacture and low in cost, is applied to the detection of the milk and milk products, can simplify operation and shorten detection time, can specifically adsorb AFM1 and AFM2, and has excellent adsorption effect; impurities in sampleintroduction are greatly reduced after being purified by the special purification column; and the special purification column has extremely strong practical popularization significance.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165BHigh luminous intensityWide excitation spectrumMaterial analysisCritical illnessUrine sample
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Detection method of gizzerosine in fish meal
ActiveCN105092766AHigh sensitivityImprove accuracyComponent separationNeutral Amino AcidsPre treatment
The invention discloses a detection method of gizzerosine in fish meal, and belongs to the technical field of detection of gizzerosine. The method disclosed by the invention comprises the following steps: sample pretreatment; enrichment and purification; on-line automated derivatization procedure. According to the detection method, gizzerosine combined in protein is extracted through sample pretreatment, and other purities and some acidic and neutral amino acid in fish meal are removed through enrichment and purification, so that separation is avoided from being disturbed, and the accuracy of detection of gizzerosine in fish meal is improved; besides, the sensitivity is high, the minimum detectable quantity is 1 mg / kg, and the detection method is suitable for detection of large-scale samples.
Owner:海南威尔检测技术有限公司 +2
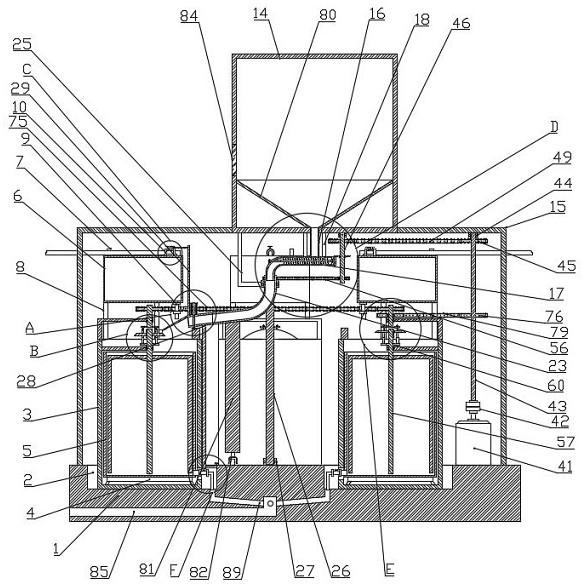
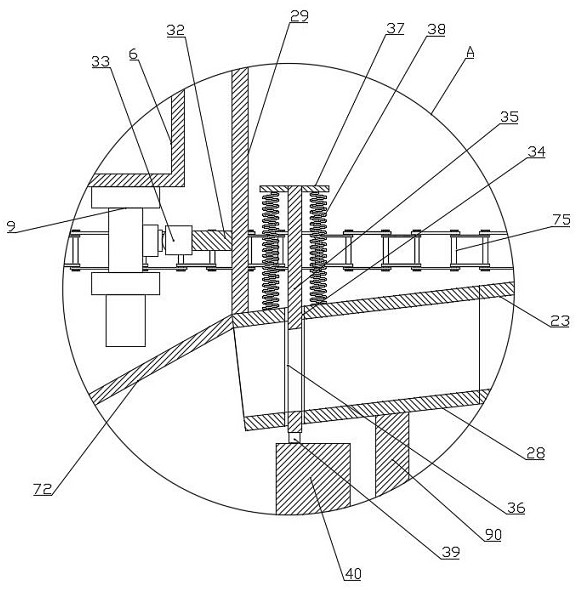
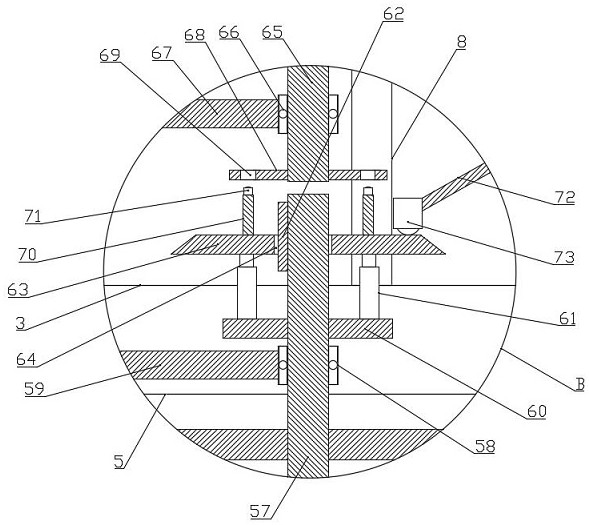
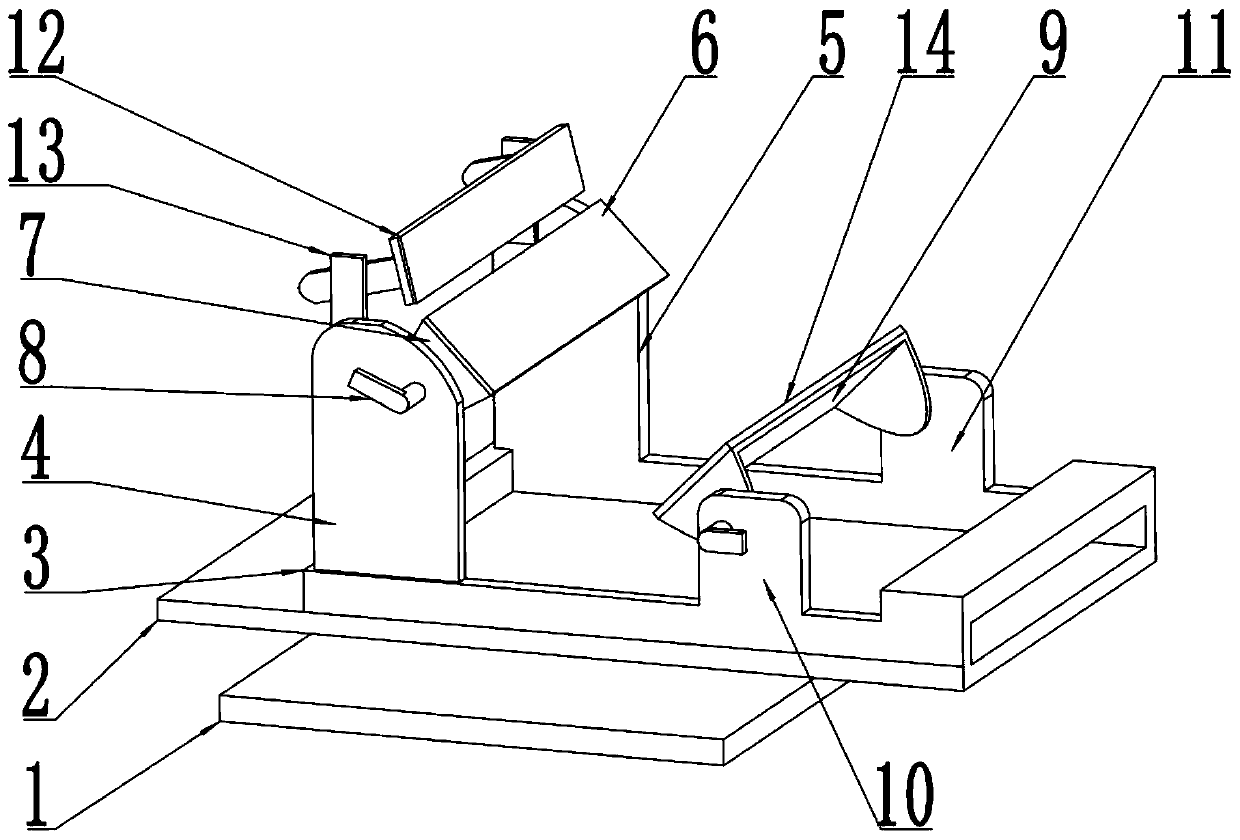
Quantitative feeding mechanism for roasting machine
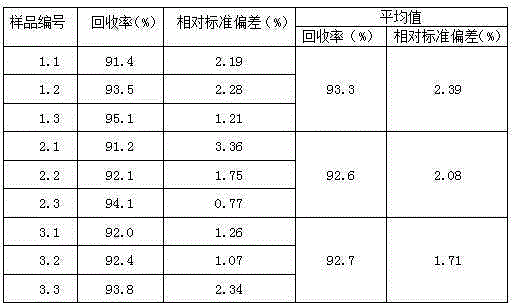
InactiveCN108455268APrevent fallingConducive to accurate quantificationConveyor partsFood treatmentDrive motor
The invention discloses a quantitative feeding mechanism for a roasting machine. The quantitative feeding mechanism comprises a base. Vertical plates are welded to the two sides of the top of the base, and the two vertical plates are symmetrically arranged. The same top plate is welded to the tops of the two vertical plates. A roasting machine body is fixedly arranged on the top of the base and located between the two vertical plates. A first installing groove is formed in the bottom of the top plate and internally provided with a drive motor. A rotation shaft is welded to an output shaft of the drive motor. A quantitative feeding mechanism body is arranged at the bottom end of the rotation shaft and matched with the roasting machine body. The quantitative feeding mechanism body comprisesquantitative columns welded to the bottom end of the rotation shaft. The quantitative feeding mechanism is simple in structure, convenient to install, economical and practical. Through a push rod motor and a baffle, materials are prevented from falling down from the interiors of quantitative holes; and meanwhile, through the quantitative columns and the quantitative holes, accurate material quantification is convenient, and material conveying is convenient.
Owner:宋明勤
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192BHigh luminous intensityWide excitation spectrumBiological testingCreatine kinaseFluorescence
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
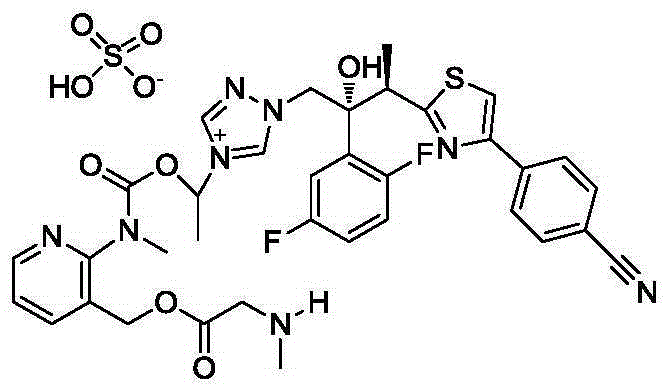
Cobicistat pamoic acid salt and preparation method therefor, drug composition and application
ActiveCN105732538ALow hygroscopicityImprove long-term stabilityOrganic active ingredientsOrganic chemistryChemistryHuman immunodeficiency virus (HIV)
The invention relates to a synergist of anti-HIV medicines, i.e., a Cobicistat pamoic acid salt and an amorphous compound thereof. The invention also relates to a preparation method of the Cobicistat pamoic acid salt and the amorphous compound thereof, a drug composition of the Cobicistat pamoic acid salt and the amorphous compound thereof and an application of the Cobicistat pamoic acid salt and the amorphous compound thereof or the drug composition in producing drugs for the treatment of HIV infection.
Owner:SOLIPHARMA
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceQuantum dot
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay kit for quantitatively detecting heart fatty acid binding protein
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
System for quantitatively detecting heavy metal cadmium and preparation method thereof
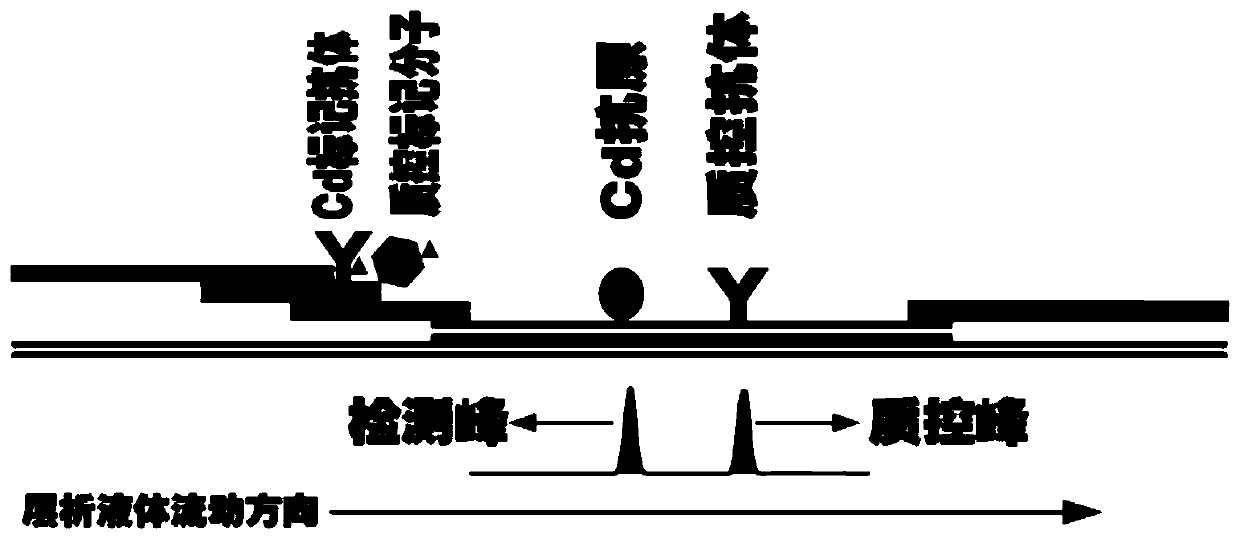
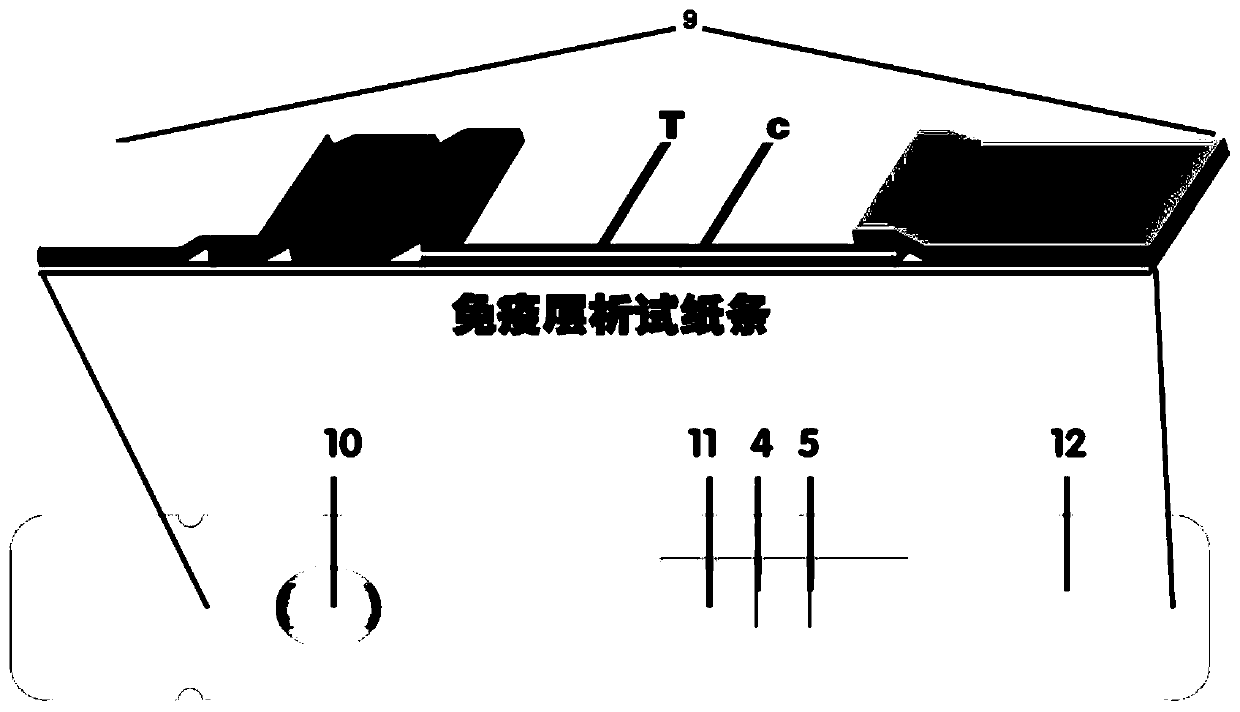
PendingCN111198264AHigh luminous intensityWide excitation spectrumMaterial analysisSample dilutionHeavy metals
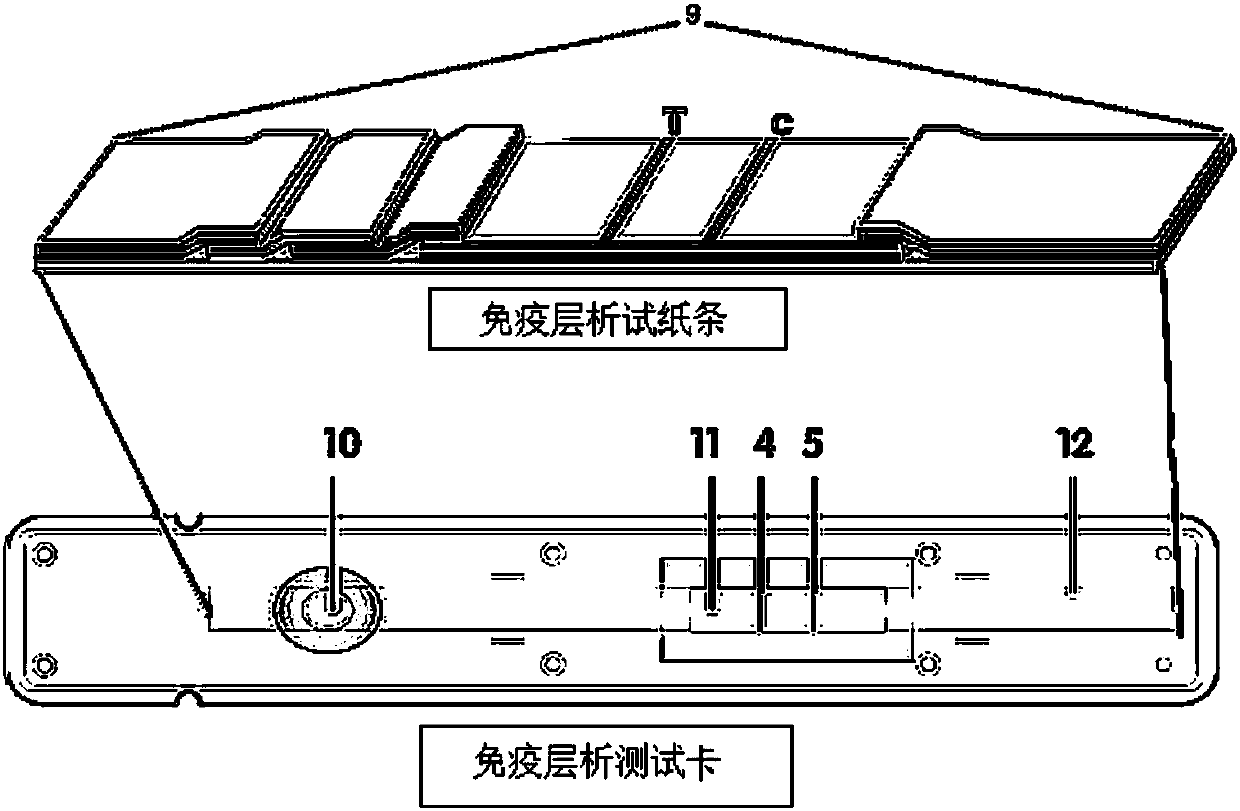
The invention provides a system for quantitatively detecting heavy metal cadmium, which comprises a buckle, a fluorescence immunochromatography test strip and a sample dilution buffer solution, wherein the buckle is of an outer shell structure of the fluorescence immunochromatography test strip and is provided with a sample adding hole and an observation window; the fluorescence immunochromatography test strip comprises a sample pad, a marking pad, a chromatography membrane, a water absorption pad and a bottom plate; a fluorescent dye modified heavy metal cadmium specific antibody and a fluorescent dye modified quality control molecule are fixed on the marking pad at the same time; a heavy metal cadmium chelating agent hapten is fixed on the quantitative detection line; the hapten is specifically combined with a fluorescent dye modified heavy metal cadmium specific antibody fixed on the marking pad; biomolecules capable of being specifically combined with the quality control moleculesare fixed on the quality control line; and a to-be-detected sample is treated with the sample dilution buffer solution before detection. In order to detect the heavy metal cadmium in the sample, treatment by using the sample dilution buffer solution is carried out before the to-be-detected sample is detected, and the sample dilution comprises a chelating agent which is combined with the heavy metal cadmium to form a heavy metal cadmium chelating agent hapten; if the sample dilution buffer solution is not used for treating a sample, the sample is directly detected, the heavy metal cadmium in the sample cannot be detected, and the existence of the sample dilution buffer solution is crucial to the detection of the cadmium in the sample.
Owner:北京大弘生物技术有限公司
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
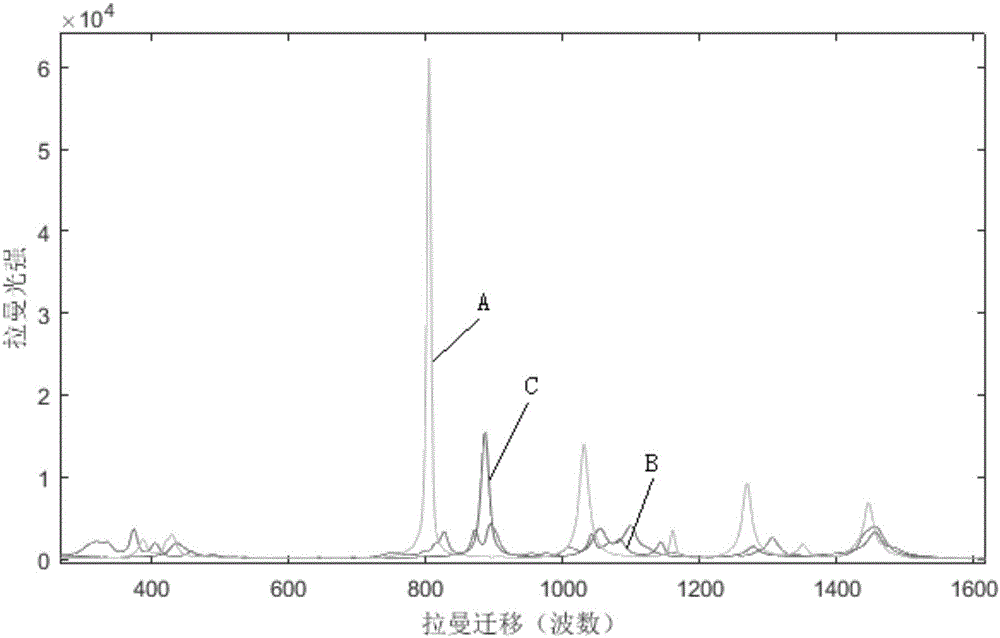
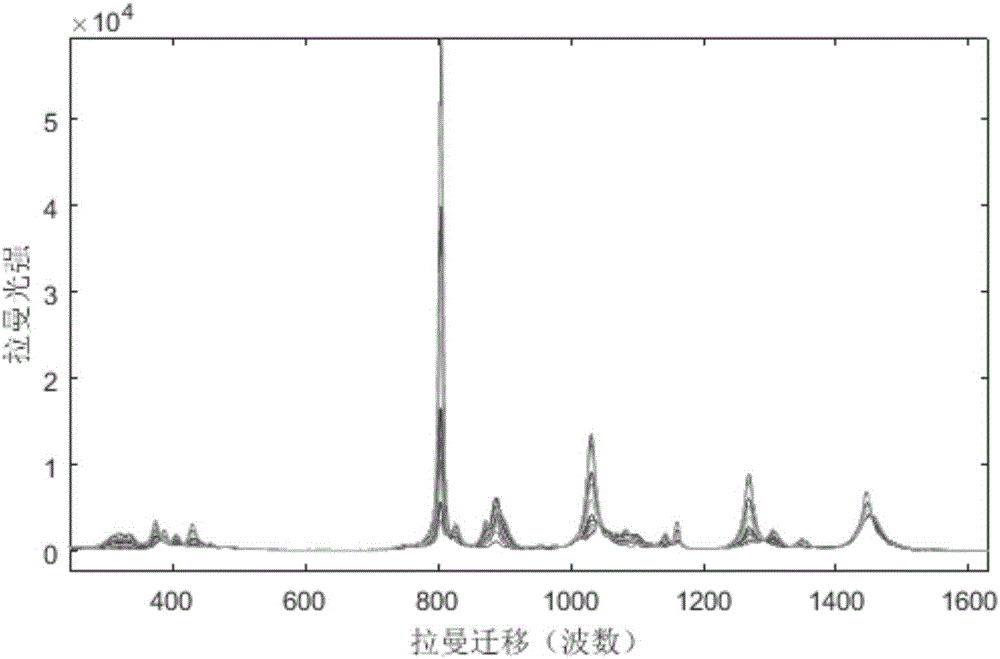
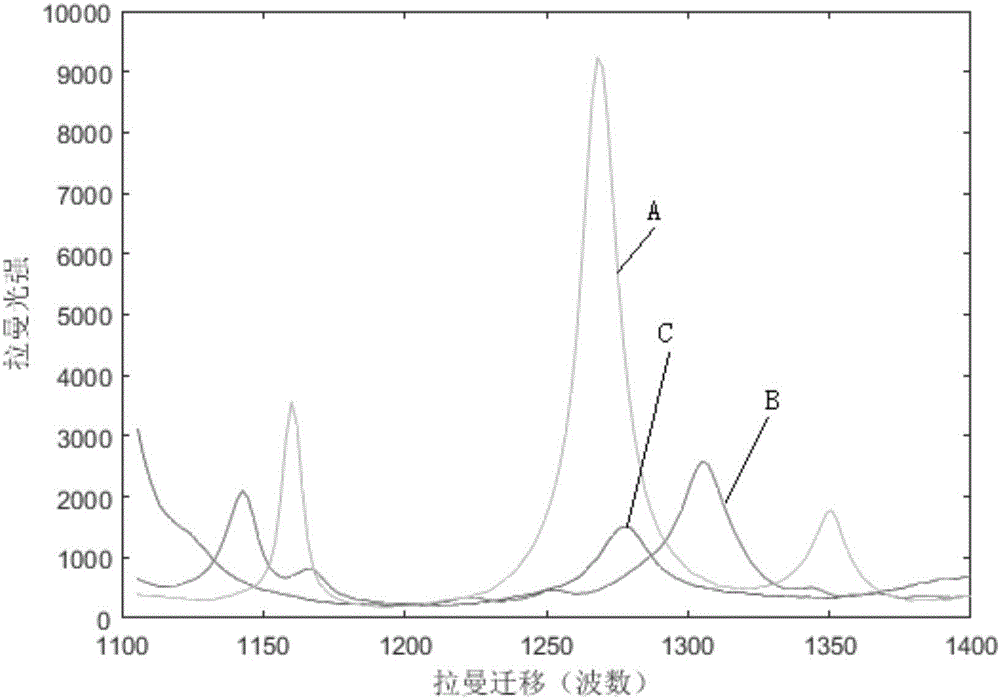
Method for directly determining components in multicomponent system adopting raman spectrum
ActiveCN106323939AConvenient for direct measurementSimple and fast operationRaman scatteringUnit operationProcess conditions
The invention discloses a method for directly determining components in a multicomponent system adopting a raman spectrum, and relates to a measuring method of the components in the multicomponent system. The method comprises the steps that 1, the raman spectrums is measured, wherein the raman spectrum of a pure solution of each component in the multicomponent system to be tested and the raman spectrum of a homogeneous solution M of the unknown-content multicomponent system to be tested are respectively measured; 2, each raman spectrum is converted to an integral spectrum through a computer, wherein the integral spectrum of the component to be quantified is recorded as SI, the matrix formed by the integral spectrums of the other components is recorded as SJ, and the integral spectrum of the homogeneous solution M is recorded as SPI; 3, the vector quantity-space included angle theta between SI and SJ is calculated, and the vector quantity-space included angle beta between SPI and SJ is also calculated; 4, the content of the component to be quantified in the homogeneous solution M is obtained through calculating the ratio of beta and theta. According to the method, direct measurement of each phase in the multicomponent system can be realized conveniently, the operation is simple and the data is real, moreover, the method can be used for the phase equilibrium study on a liquid-liquid multicomponent system and process condition control of a series of separation unit operations such as extraction and rectification.
Owner:OPTOTRACE SUZHOU TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com