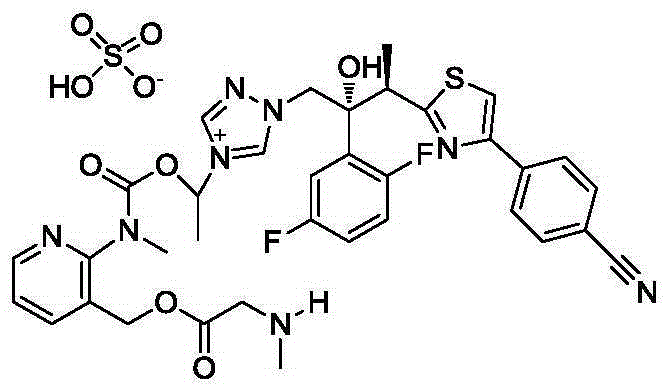
Isavuconazole sulfate crystal and preparation method thereof
A technology of isavuconazole sulfate and crystals, which is applied in the field of antifungal drug isavuconazole sulfate crystal form and its preparation, and can solve the problems of stability, water-soluble dissolution rate, storage stability, preparation easiness, Affect drug activity and other issues, to achieve the effect of stability, small change in purity, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of isavuconazole sulfate crystal form
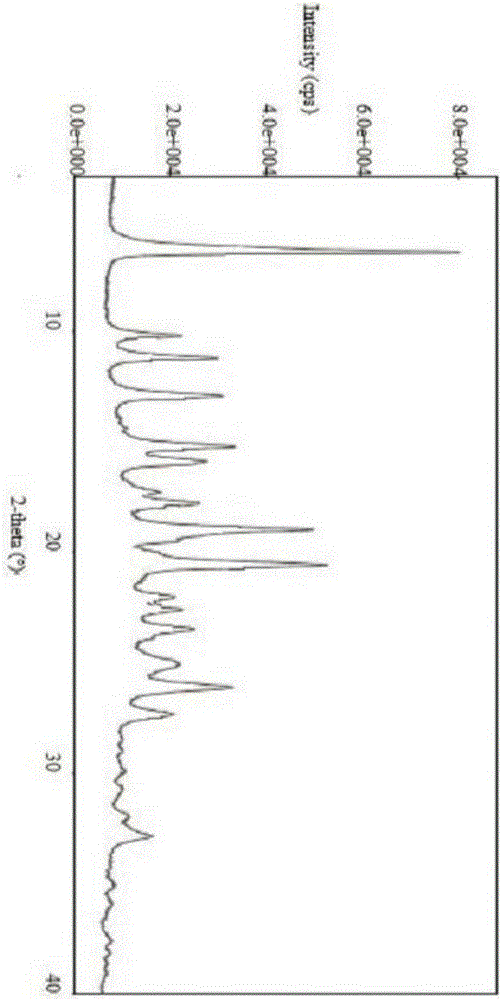
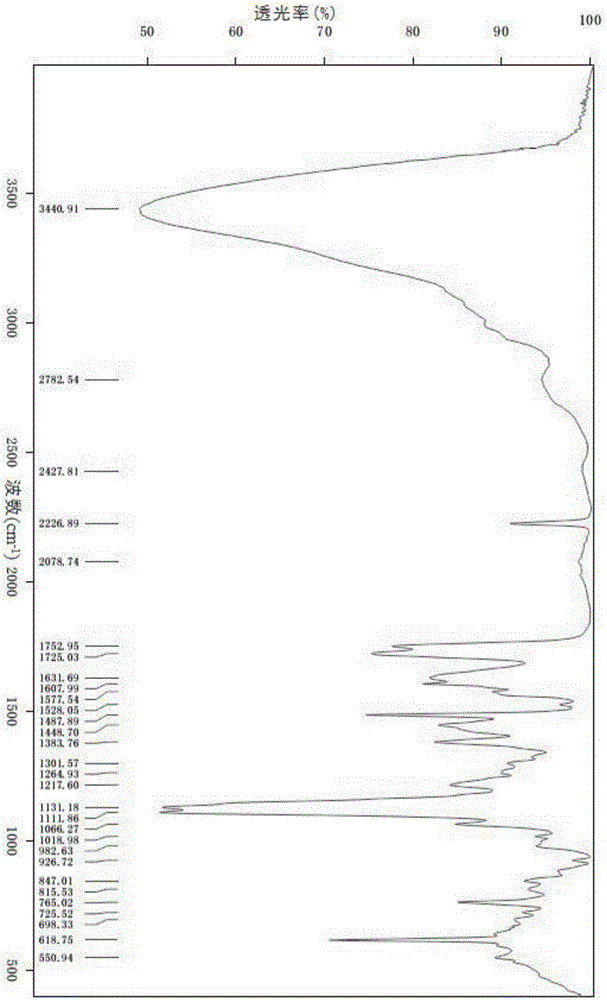
[0039]Dissolve 0.9 g (1 mmol) of isavuconazole hydrochloride in 9 mL of water, lower the temperature to 0 ° C, adjust the pH to 7 with aqueous sodium bicarbonate solution, add 9 mL of dichloromethane for extraction, dry and concentrate after liquid separation, and then add 10 mL of methanol Dissolve, cool down to -5°C, add 0.09g (1mmol) of concentrated sulfuric acid and 0.11g (1mmol) of 30% hydrogen peroxide dropwise, stir for 30min, concentrate, add 5mL of 95% ethanol and 5mL of ethyl acetate for recrystallization, and heat to 40 ℃ dissolved, stirred and cooled to 20 ℃, crystallized, suction filtered, and vacuum dried to obtain 0.62g white solid with a yield of 76% and a purity of 97.6%. The XRPD pattern was as follows: figure 1 shown.
Embodiment 2
[0040] Embodiment 2 Preparation of isavuconazole sulfate crystal form
[0041] Dissolve isavuconazole hydrochloride 0.9g (1mmol) in 9mL of water, cool down to -5°C, adjust the pH to 7 with ammonia solution, add 9mL of dichloromethane for extraction, dry and concentrate after liquid separation, add 10mL of methanol to dissolve , cooled to -5°C, added dropwise 0.12g (1.2mmol) of concentrated sulfuric acid and 0.15g (1.3mmol) of 30% hydrogen peroxide, stirred for 30min, concentrated, added 5mL of 95% ethanol and 10mL of ethyl acetate for recrystallization, and heated to Dissolve at 40°C, stir and cool to 10°C, crystallize, filter with suction, and dry in vacuo to obtain 0.55 g of white solid with a yield of 67% and a purity of 97.4%.
Embodiment 3
[0042] Embodiment 3 Preparation of isavuconazole sulfate crystal form
[0043] Dissolve 0.9 g (1 mmol) of isavuconazole hydrochloride in 9 mL of water, cool down to -3 °C, adjust the pH to 7 with aqueous sodium carbonate solution, add 9 mL of dichloromethane for extraction, dry and concentrate after liquid separation, then add 10 mL of ethanol Dissolve, cool down to -5°C, add 0.13g (1.3mmol) of concentrated sulfuric acid and 0.12g (1.1mmol) of 30% hydrogen peroxide dropwise, stir for 30min, concentrate, add 5mL of 95% ethanol and 20mL of ethyl acetate for recrystallization, and heat Dissolve at 40°C, stir and cool to 20°C, crystallize, filter with suction, and dry in vacuo to obtain 0.50 g of white solid with a yield of 62% and a purity of 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com