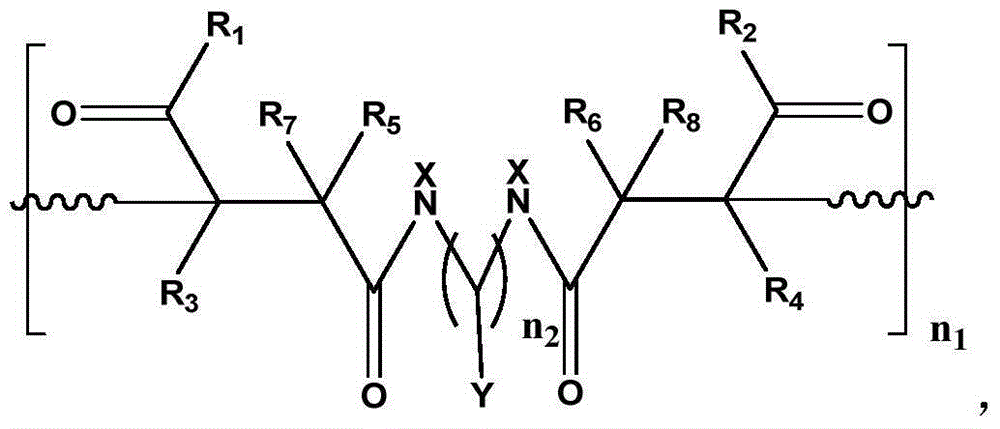
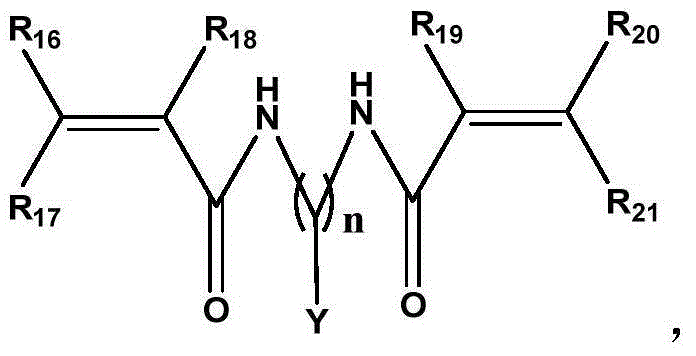
Macroporous crosslinked antibacterial macromolecular resin containing halamine functional group, as well as preparation and application thereof
A polymer resin and cross-linked polymer technology, applied in the fields of application, botanical equipment and methods, biocides, etc., can solve the problems of high production cost, poor surface hydrophilicity, etc., and achieve enhanced surface hydrophilicity and enhanced antibacterial Efficacy, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Present embodiment is the preparation (I) of polymethacrylamide-methylenebisacrylamide macroporous cross-linked resin, and its reaction formula is as follows:
[0042]
[0043] Water phase: Take 2.4g of methacrylamide (MAA), 0.8g of methylenebisacrylamide (MBAA), 0.25g of potassium persulfate (PPS), and 2ml of N,N-dimethylformamide (DMF) into Into a 50ml single-necked flask, add 14ml of deionized water and stir to dissolve, then heat at a constant temperature of 40°C for later use.
[0044] Oil phase: Add 36ml of cyclohexane into a 100ml three-necked flask, then add a small amount of emulsifier OP-4, heat at a constant temperature of 40°C under mechanical stirring for later use.
[0045] After the water phase is dissolved, add the water phase to the oil phase drop by drop, keep the temperature for 0.5h after the addition is completed, then set the temperature to 70°C, adjust the speed to 700r / min, and reflux for 3h to obtain the prepared Polymethacrylamide-methylene...
Embodiment 2
[0048] This embodiment is the preparation (II) of polymethacrylamide-methylenebisacrylamide macroporous cross-linked resin
[0049] Water phase: Take 1.0g of methacrylamide (MAA), 1.0g of methylenebisacrylamide (MBAA), 0.15g of potassium persulfate (PPS), and 2ml of N,N-dimethylformamide (DMF) into Into a 50ml single-necked flask, add 14ml of deionized water and stir to dissolve, then heat at a constant temperature of 40°C for later use.
[0050] Oil phase: Add 36ml of cyclohexane into a 100ml three-necked flask, then add a small amount of emulsifier OP-4, heat at a constant temperature of 40°C under mechanical stirring for later use.
[0051] After the water phase is dissolved, add the water phase to the oil phase drop by drop, keep the temperature for 0.5h after the addition is completed, then set the temperature to 70°C, adjust the speed to 700r / min, and reflux for 3h to obtain the prepared Polymethacrylamide-methylenebisacrylamide macroporous cross-linked resin.
[0052]...
Embodiment 3
[0055] This example is the preparation of polyacrylic acid-methylenebisacrylamide macroporous cross-linked resin, and its reaction formula is as follows:
[0056]
[0057] Water phase: Take 1.0g of acrylic acid (AA), 1.0g of methylenebisacrylamide (MBAA), 0.15g of potassium persulfate (PPS), and 2ml of N,N-dimethylformamide (DMF) into a 50ml single-necked flask Add 14ml of deionized water and stir to dissolve, then heat at 40°C for later use.
[0058] Oil phase: Add 36ml of cyclohexane into a 100ml three-necked flask, then add a small amount of emulsifier OP-4, heat at a constant temperature of 40°C under mechanical stirring for later use.
[0059] After the water phase is dissolved, add the water phase to the oil phase drop by drop, keep the temperature for 0.5h after the addition is completed, then set the temperature to 70°C, adjust the speed to 700r / min, and reflux for 3h to obtain the prepared Polyacrylic acid-methylenebisacrylamide macroporous cross-linked resin.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com