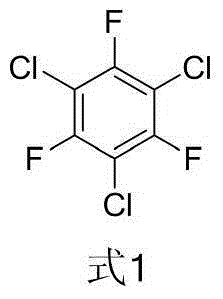
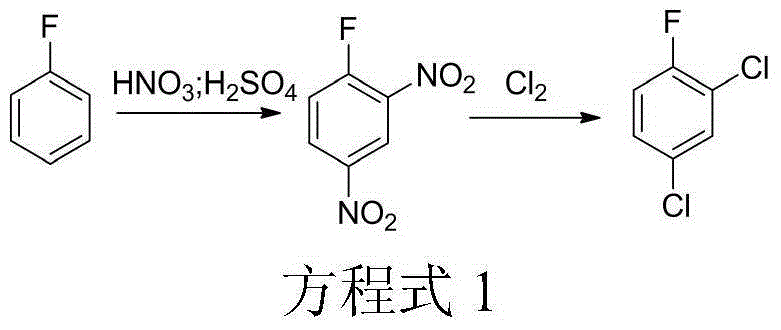
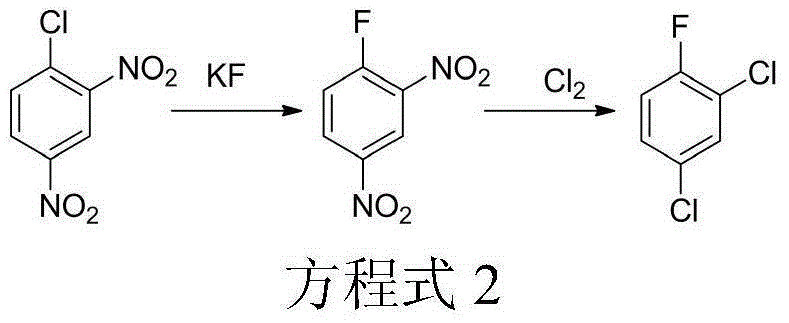
Method for synthesizing 1,3,5-trichloro-2,4,6-trifluorobenzene from 2,4-difluoro-3,5-dichloronitrobenzene
A technology of dichloronitrobenzene and trifluorobenzene, applied in the field of organic synthesis, can solve the problems of group rearrangement isomerization, high fluorination reaction temperature, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of 1,3,5-trichloro-2,4-difluorobenzene:
[0054] Weigh 228.0g of 2,4-difluoro-3,5-dichloronitrobenzene, and throw it into a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, a ventilation duct, an air condenser, a receiving bottle and an exhaust gas absorption device, and heat up To 195°C, slowly pass chlorine gas under stirring, fix the chlorine gas flow rate at 4000L / h, rectify, stop the reaction when no more fractions flow out, adjust the pH of the fractions to 7 with 10% sodium bicarbonate aqueous solution, separate the organic layer, The organic layer was dried with anhydrous sodium sulfate and filtered to obtain 210.0 g of the product 1,3,5-trichloro-2,4-difluorobenzene, with a yield of 96.5% and a GC purity of 99%.
[0055] Same as embodiment 1 mode of operation, change the temperature of chlorination reaction, chlorine flow and reaction solvent in the chloride synthesis process, the results are shown in table 1:
[0056] Table 1...
Embodiment 7
[0059] Synthesis of 2,4,6-trichloro-3,5-difluoronitrobenzene:
[0060] Put 200g of sulfuric acid into the flask, then dropwise add 100g of 1,3,5-trichloro-2,4-difluorobenzene prepared in Example 1 into the flask, start stirring, raise the temperature to 80°C, and start to drop 59g A mixed acid solution with a mass ratio of sulfuric acid and nitric acid of 1:1; the dosage of nitric acid in the mixed acid solution is equivalent to 1.0 times the molar weight of 2,4,6-trichloro-3,5-difluoronitrobenzene After the completion, keep the reaction for 2 hours; after the reaction, spontaneously ignite and cool down and let it stand for 30 minutes, then start to separate layers, adjust the pH of the upper layer to 7 with aqueous sodium bicarbonate solution, dry the organic layer with anhydrous sodium sulfate, and filter to obtain the product 2,4 , 118.0 g of 6-trichloro-3,5-difluoronitrobenzene, the yield was 97.8%, and the GC purity of 2,4,6-trichloro-3,5-difluoronitrobenzene was 94%.
...
Embodiment 13
[0066] Synthesis of 1,3,5-trichloro-2,4,6-trifluorobenzene:
[0067] Throw 100g of 2,4,6-trichloro-3,5-difluoronitrobenzene prepared in Example 7 into the flask, heat up for dehydration, stop heating when the temperature reaches 140°C, and put in 30g of potassium fluoride and 2g Tetra-n-butylammonium chloride, heated up to 160°C for secondary dehydration, reacted at the reaction temperature for 4 hours, after the reaction was completed, washed with heated water, stirred for 30 minutes, left to separate and separated, and the materials were distilled to obtain the product 1,3 , 5-trichloro-2,4,6-trifluorobenzene 85.5g, yield 95.3%, GC purity 96%.
[0068] The difference from Example 13 is that the reaction temperature during the fluorination reaction, the reaction solvent, the reaction time, the equivalent of potassium fluoride added, the results are shown in Table 3:
[0069] table 3
[0070]
[0071] As can be seen from Table 3, the reaction temperature and the feeding a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com