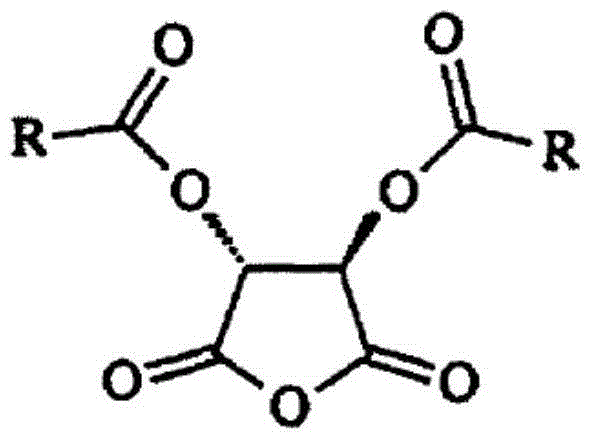
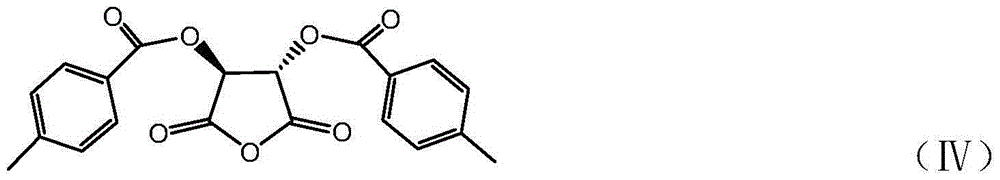
Synthetic method for eslicarbazepine acetate
A technology of eslicarbazepine acetate and synthesis method, which is applied in the field of drug synthesis, can solve the problem of insufficient ee value, and achieve the effect of simplifying the operation steps and improving the yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 110
[0035] Example 110-Hydroxy-10,11-dihydro-5H-dibenzo[b,f]azepine -Preparation of 5-carboxamide (licarbazepine, Ⅲ)
[0036] In a 50L reaction kettle equipped with an electric stirrer, add 3.0kg (11.9mol) of oxcarbazepine, 14L of 95% ethanol and 7L of purified water, and stir to dissolve it. Slowly add 360g (9.13mol) of sodium borohydride to the mixed solution in batches at 10-15°C (completely added in about 1 hour), continue to stir and react for 10 minutes, then slowly raise the temperature to 45°C, and keep the reaction for 2 hours. TLC detection (ethyl acetate: methanol = 2:3), after the reaction was completed, the reaction solution was cooled to 10°C-15°C, and 5.2L of acetone was slowly added. The mixture was distilled to near dryness at 40°C under reduced pressure, stirred and added with 13L of purified water, a large amount of solids were precipitated, and crystallized by cooling. After filtering and washing (2×1.5 L of water), the resulting solid was dried at 100°C for...
Embodiment 2
[0037] Example 2 (2S,3S)-4-[((S)-5-carbamoyl-10,11-dihydro-5H-dibenzo[b,f]azepine Preparation of -10-yl)oxy]-2,3-bis((4-methylbenzoyl)oxy)-4-oxobutanoic acid (V)
[0038] In the reaction kettle equipped with electric stirrer, add 15L dichloromethane, 2.21kg (8.7mol) licarbazepine, 47g (0.38mol) DMAP, D-p-methyldibenzoyl tartaric anhydride 3.64kg (9.88 mol) was stirred at room temperature for 30 min, and 635 g (8.03 mol) of pyridine was added dropwise. The mixture was stirred and reacted at 20°C for 24 hours, and detected by TLC (ethyl acetate:methanol=3:2). After the reaction, the mixture was washed with water (10L×2), and the organic layer was evaporated to dryness under reduced pressure to obtain 5kg of white powder. The obtained powder was dissolved by heating with 11L of acetonitrile, stirred for 1 h, and frozen in the refrigerator for about 12 h (-7°C). The precipitated solid was suction filtered, and the filter cake was dried to obtain a white solid (2S,3S)-4-[((S)-5-...
Embodiment 3
[0039] Example 3 (S)-10-hydroxyl-10,11-dihydro-5H-dibenzo[b,f]azepine -Preparation of 5-carboxamide (eslicarbazepine, VI)
[0040] In a three-neck flask equipped with an electric stirrer, add 1.8kg (2.91mol) of V, 3L of methanol, 3L of water, and 140g of NaOH, and stir and react for 2 hours at room temperature. TLC detection (ethyl acetate: methanol = 2:3), after the reaction, filter, wash (2×1L methanol), collect the filtrate, concentrate and distill methanol at about 45°C. 2L of water was added to the residue at 10-15°C, a large amount of solids were precipitated, and crystallized by cooling. Filter, wash (2×1L water), and dry the obtained solid at 45°C-50°C for 2 hours, then raise the temperature to 100°C and dry for 6-8 hours to obtain 628g of white solid LKXP-Ⅵ, with a yield of 85% and an ee value of 96.7% , melting point 186°C-187°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com