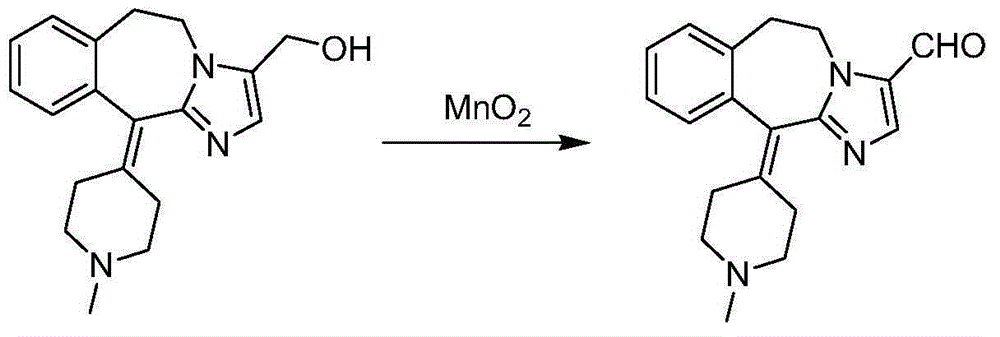
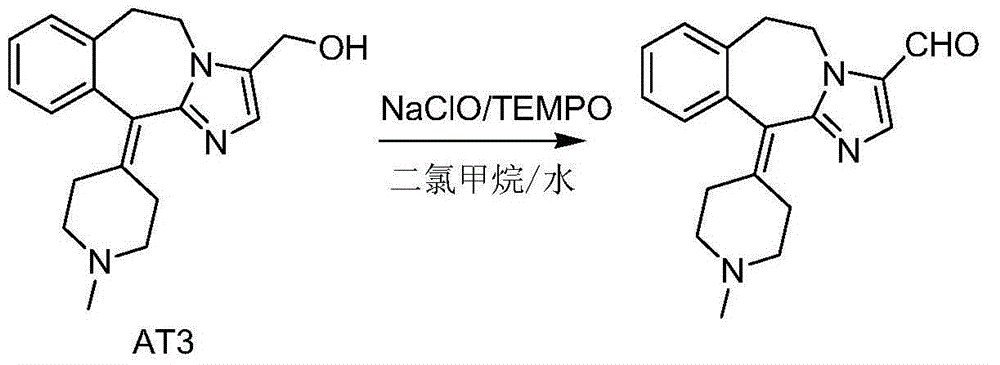
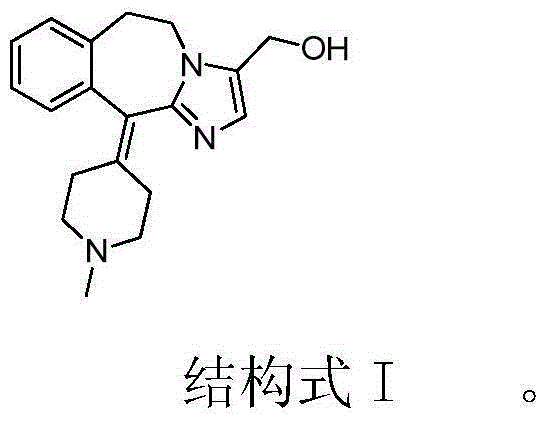
Novel oxidation method for synthesis of alcaftadine
A technology of alcastatine and oxidizing agent, which is applied in the field of drug preparation, can solve the problems of unsuitability for industrial scale-up production, polycarboxylic acid impurities, high price and the like, and achieves the effects of poor product purity, high total yield and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add AT330.9g (0.1mol), TEMPO0.16g (1mmol), and 300mL dichloromethane into a 500mL three-necked flask, stir to dissolve, add potassium bromide solution (1.19g potassium bromide (10mmol) dissolved in 6mL purified water), Stir and cool down to -10~-5°C, add 53mL (0.15mol) of 10% sodium hypochlorite solution dropwise, after dropping, keep stirring at -10~-5°C for 30min, raise the temperature to 10~20°C, stir for 30min, monitor by TLC until the reaction is complete , standing for stratification, separating the organic layer, washing the organic layer twice with 100mL sodium bicarbonate solution, separating the organic layer, adding anhydrous sodium sulfate for drying, suction filtration, and concentrating the filtrate under reduced pressure. The obtained oil was added with 50 mL of isopropanol and stirred to precipitate a solid, which was filtered and dried to obtain 26.5 g of an off-white solid with a yield of 86.3%. HPLC purity: 98.7%.
Embodiment 2
[0029] Add AT330.9g (0.1mol), TEMPO0.16g (1mmol), and 300mL dichloromethane into a 500mL three-neck flask, stir to dissolve, add sodium bromide solution (1.03g sodium bromide (10mmol) dissolved in 15mL purified water), Stir and cool down to -10~-5°C, add 106mL (0.15mol) of 5% sodium hypochlorite solution dropwise, after dropping, keep stirring at 20~30°C for 30min, raise the temperature to 10~20°C, stir for 30min, monitor by TLC until the reaction is complete, statically Separate the layers, separate the organic layer, wash the organic layer twice with 100 mL of sodium bicarbonate solution, separate the organic layer, add anhydrous sodium sulfate to dry, filter with suction, and concentrate the filtrate under reduced pressure. The obtained oil was added with 50 mL of isopropanol and stirred to precipitate a solid, which was filtered and dried to obtain 24.6 g of an off-white solid with a yield of 80.1%. HPLC purity: 98.9%.
Embodiment 3
[0031] Add AT330.9g (0.1mol), TEMPO0.16g (1mmol), and 300mL dichloromethane into a 500mL three-neck flask, stir to dissolve, add potassium iodide solution (1.66g potassium iodide (10mmol) dissolved in 15mL purified water), stir and cool down to - 10~-5℃, add 71mL (0.2mol) of 10% sodium hypochlorite solution dropwise, after dropping, keep stirring at -10~-5℃ for 30min, raise the temperature to 10~20℃, stir for another 30min, monitor by TLC until the reaction is complete, let stand Separate the layers, separate the organic layer, wash the organic layer twice with 100 mL of sodium bicarbonate solution, separate the organic layer, add anhydrous sodium sulfate to dry, filter with suction, and concentrate the filtrate under reduced pressure. The obtained oil was added with 50 mL of isopropanol and stirred to precipitate a solid, which was filtered and dried to obtain 26.2 g of an off-white solid with a yield of 85.3%. HPLC purity: 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com