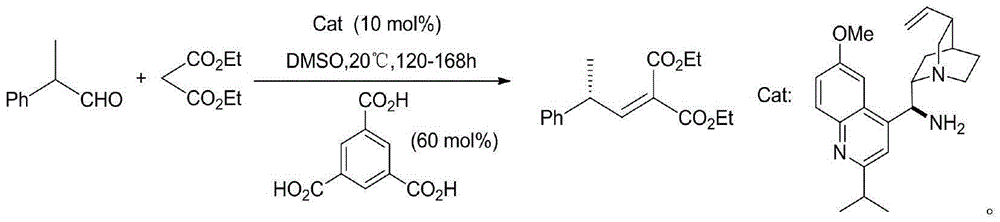
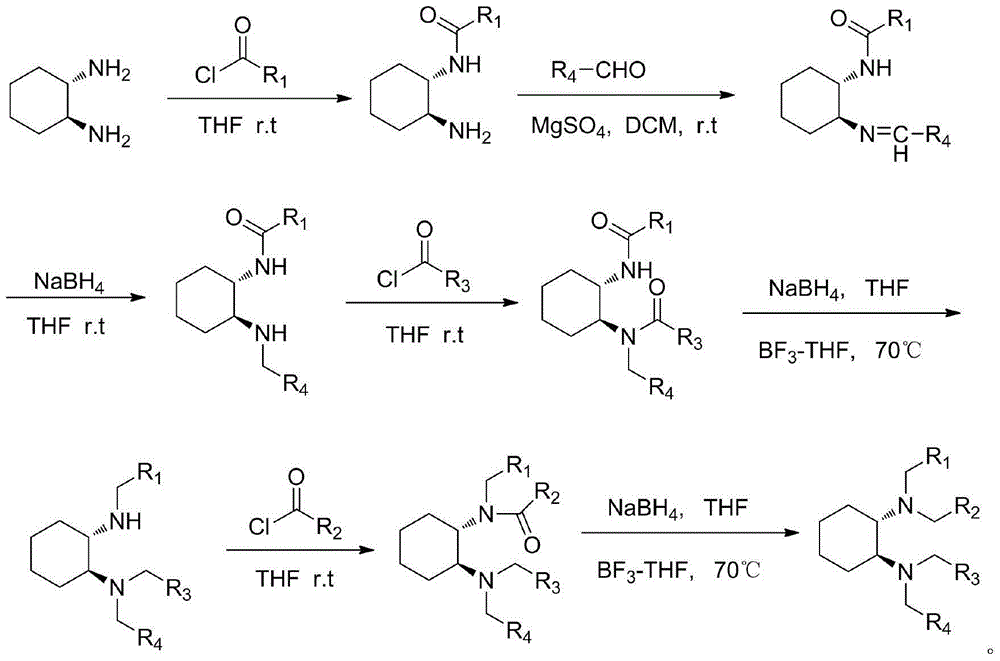
Method of catalyzing asymmetric Knoevenagel reaction via trans-1, 2-diaminocyclohexane derivative
A technology of cyclohexanediamine and derivatives, which is applied in the field of synthesis of organic intermediates, can solve the problems of insufficient catalytic effect, low substrate universality, etc., and achieves high steric selectivity and enantioselectivity and improved yield. , the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of diethyl 2-(2-phenylpropylene) malonate, the synthetic route is:
[0035]
[0036] 2-Phenylpropanal (0.001mol, 0.134g), diethyl malonate (0.0011mol, 0.176g) and pre-prepared Cat (0.0001mol, 0.032g), acetonitrile (2mL) as a solvent, stirred and reacted at 60°C for 168h under a nitrogen atmosphere, then stopped the reaction, added a small amount of 0.1mol / L dilute hydrochloric acid (10mL) to wash the reaction system after cooling, and extracted the aqueous phase three times with ethyl acetate (3× 15mL), the organic phases were combined, and the organic phases were sequentially washed with saturated NaHCO 3 solution (10mL), saturated brine (15mL), washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was rotary evaporated under reduced pressure to remove the solvent to obtain a crude product, which was separated by column chromatography to obtain 0.221 g of the target product, with an ee value of 73% and a yield of 80%.
[0037] 1 ...
Embodiment 2
[0039] The synthesis of diethyl 2-(2-(4-fluorophenyl) propylene) malonate, the synthetic route is:
[0040]
[0041] Add p-fluoro 2-phenylpropanal (0.001mol, 0.152g), diethyl malonate (0.0011mol, 0.176g) and pre-prepared Cat (0.0001mol, 0.032g) into a 10mL reaction tube in sequence , acetonitrile (2mL) was used as a solvent, and the reaction was stirred at 60°C for 168h under a nitrogen atmosphere, and then the reaction was stopped. After the reaction system was cooled, a small amount of 0.1mol / L dilute hydrochloric acid (10mL) was added for washing, and the aqueous phase was extracted three times with ethyl acetate ( 3×15mL), the organic phases were combined, and the organic phases were sequentially washed with saturated NaHCO 3 solution (10mL), saturated brine (15mL), washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was rotary evaporated under reduced pressure to remove the solvent to obtain a crude product, which was separated by column chromato...
Embodiment 3
[0044] The synthesis of diethyl 2-(2-(4-methoxyphenyl) propylene) malonate, the synthetic route is:
[0045]
[0046] In a 10mL reaction tube, add p-methoxy 2-phenylpropanal (0.001mol, 0.164g), diethyl malonate (0.0011mol, 0.176g) and pre-prepared Cat (0.0001mol, 0.017 g), acetonitrile (2mL) was used as a solvent, and the reaction was stirred at 60°C for 168h under a nitrogen atmosphere, and then the reaction was stopped. After the reaction system was cooled, a small amount of 0.1mol / L dilute hydrochloric acid (10mL) was added for washing, and the aqueous phase was extracted with ethyl acetate Three times (3×15mL), the organic phases were combined, and the organic phases were sequentially washed with saturated NaHCO 3 solution (10mL), saturated brine (15mL), washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was rotary evaporated under reduced pressure to remove the solvent to obtain a crude product, which was separated by column chromatography to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com