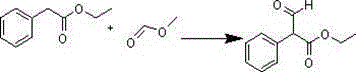Preparation method of 2-phenyl-1,3-propanediol
A technology of propylene glycol and phenyl, which is applied in the preparation of hydroxyl compounds, organic compounds, and carboxylate esters, can solve the problems of high cost of preparation methods and complicated post-treatment processes, and achieve remarkable technological progress, great application value, and The effect of high product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1α-formylphenylacetic acid ethyl ester
[0024] a. Add 150mL anhydrous toluene, 16.4g (0.1mol) ethyl phenylacetate, 8.1g sodium methoxide solid into a 500mL four-neck flask, stir, heat, raise the temperature to 40-45°C, keep warm for 0.5h, add 8.9 g (0.15mol) of methyl formate in 50mL of toluene solution, after the dropwise addition, keep warm, and the plate reaction is completed, then cool down to -5 ~ 0°C, add 100mL of ice-water mixture dropwise to the flask under stirring, dropwise, Stand still, separate the water layer, adjust the pH to about 1-2 with 1mol / L sulfuric acid, extract with toluene (200mL×3), combine the toluene liquid, wash with saturated brine, dry with anhydrous sodium sulfate, and spin off the solvent 18g crude product , That is, ethyl α-formyl phenylacetate, the yield is 93.75%.
[0025] b. Add dried 150mL petroleum ether, 16.4g (0.1mol) ethyl phenylacetate, 8.1g sodium methoxide solid into a 500mL four-necked flask, sti...
Embodiment 22
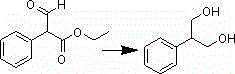
[0026] The synthesis of embodiment 22-phenyl-1,3-propanediol
[0027] a. Add 2.3g of sodium borohydride and 100mL of absolute ethanol to a 250mL four-neck flask, stir, cool down below 0°C, add 4.8g of ethyl α-formylphenylacetate in 10mL of absolute ethanol solution dropwise, and add During the process, keep the temperature of the reaction solution below 0°C. After the dropwise addition is completed, keep warm for 0.5h and then raise the temperature to 50-55°C. React, spot the plate, and drop to room temperature after the reaction is completed. Add 1mol / L sulfuric acid dropwise to the flask to adjust the pH to about 3, raise the temperature to 50-55°C, keep warm for 2 hours, spin off the solvent, add water to dissolve, extract with ethyl acetate, spin dry, and use ethyl acetate:petroleum ether (1 : 3) dissolved, cooled and suction filtered, and washed with petroleum ether to obtain 2.3g. Yield 60.5%.
[0028] b. Add 3.8g of potassium borohydride, 100mL of absolute ethanol, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com