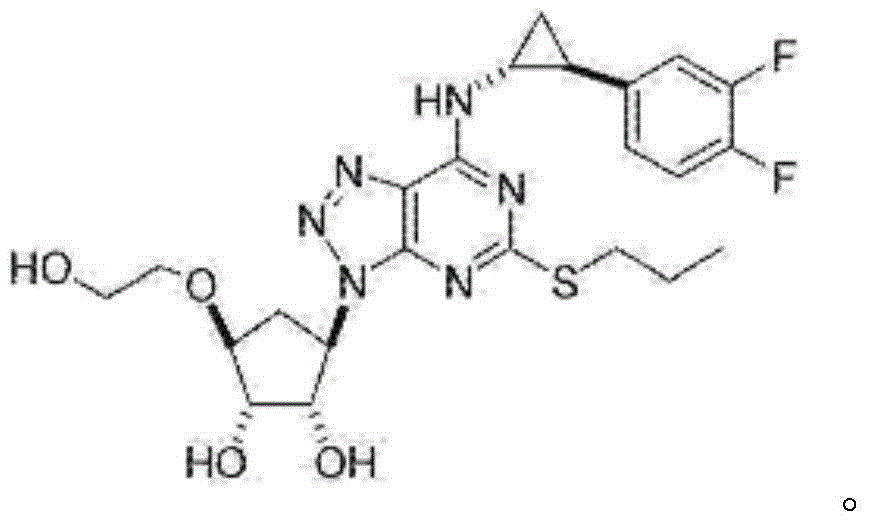
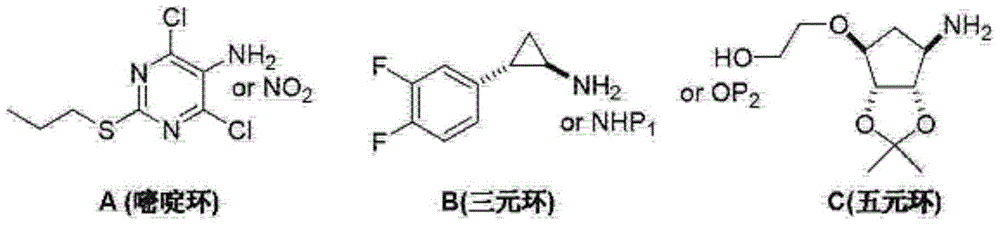
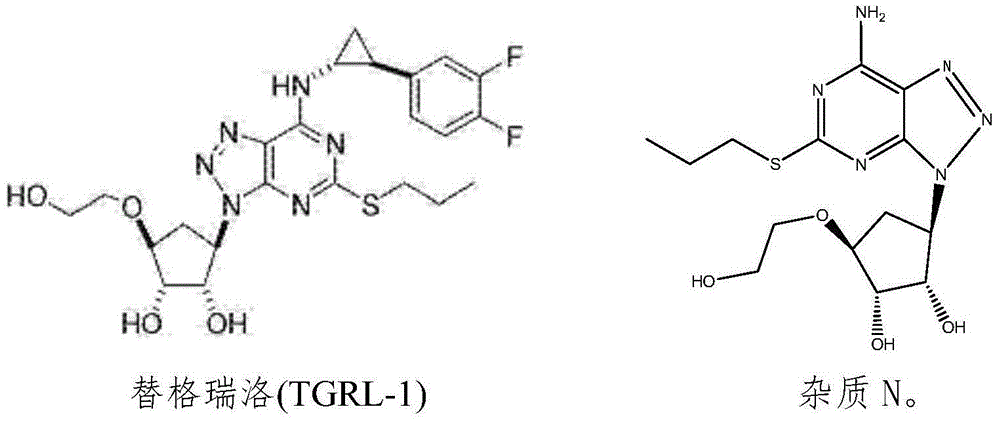
Ticagrelor crystal and medicinal composition comprising same
A ticagrelor crystallization technology, which is applied in the field of ticagrelor crystallization and pharmaceutical compositions containing the crystallization, can solve problems such as difficulty in obtaining ticagrelor products that meet the purity requirements, improve quality, increase yield, The effect of simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 "One-pot" preparation of ticagrelor
[0049] Preparation of TGRL-4
[0050]
[0051] Add TGRL-3 (7.00kg), TGRL-2 (5.46kg), ethylene glycol (19.6kg) and triethylamine (6.77kg) into the reactor respectively, heat to 100°C and stir for 8h. Cool down to room temperature, add ethyl acetate and water, stir and separate phases. The organic phase was decolorized by adding activated carbon, filtered, and the filtrate was concentrated to a solid. Add ethyl acetate and stir until the solid is completely dissolved, add n-hexane dropwise, stir for 2-3h, and filter. Dry to obtain TGRL-4 solid with a purity of 99.4% and a yield of 81.5%.
[0052] Preparation of TGRL-5:
[0053]
[0054] Add TGRL-4 (6.7kg) toluene (26.5kg) and glacial acetic acid (5.76kg) to the reaction kettle. Stir to dissolve, add dropwise an aqueous solution of sodium nitrite, and stir for 1h. Then add K dropwise to the reaction system 2 CO 3 aqueous solution. After stirring, the lower aque...
Embodiment 2
[0061] Example 2 "One-pot" preparation of ticagrelor
[0062] According to the feeding and method of Example 1, in the preparation of TGRL-4, the reaction temperature is 110°C, and the reaction time is 9h;
[0063] Preparation of TGRL-5: room temperature, reaction time: 2h;
[0064] Preparation of TGRL-6: room temperature, reaction time: 5h;
[0065] Preparation of TGRL-1: Reaction temperature: 15°C, reaction time: 4h, 5.6kg of ticagrelor was obtained, the total yield was 56%, the purity was 96.65%, and the impurity N was 1.0%.
Embodiment 3
[0066] The refined preparation of embodiment 3 ticagrelor
[0067] Crystallization of Crude TGRL-1
[0068] Add methanol (67 L) to the TGRL-1 solid obtained in Example 1, and stir to dissolve. Water (26.8 L) was added dropwise with stirring, and stirred for 10 minutes. Continue to add water (37.5L) dropwise at a rate of 5ml / s, and crystallize for 2-3h. Cooled to 0-5 ° C crystallization 0.5h. Filter and wash the filter cake with water. The solid was dried under vacuum at 30°C to obtain the crude product of TGRL-1. Yield 90%, purity 98.26%, impurity N0.1%.
[0069] Refinement of TGRL-1
[0070] The TGRL-1 crude solid (6.0 kg) from the previous step was added to the reaction kettle. Ethyl acetate (60 L) was added. Stir and heat at 40-45°C to dissolve. Add n-hexane (78L) dropwise, crystallize at room temperature for 2-3h after the dropwise addition, and then cool to 0-5°C for 0.5h. After filtering, the filter cake was double-cone dried at 30°C to obtain a refined product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com