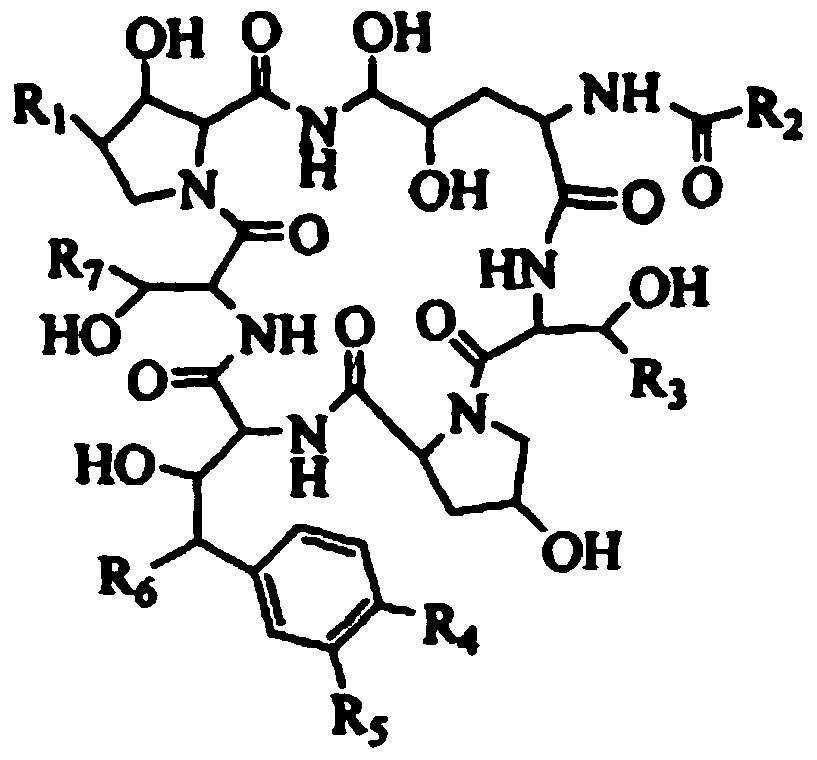
A kind of preparation method and application of immobilized cyclic lipopeptide deacylase
A technology of cyclolipopeptide deacylase and enzyme activity, which is applied in the field of preparation of immobilized cyclolipopeptide deacylase, can solve the problems of low recovery rate of immobilized enzyme activity, enzyme loss, increased cost, etc., and save enzyme purification Equipment, Enhanced Selective Binding, High Transformation Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The acquisition of embodiment 1 cyclolipopeptide deacylase solution
[0027] 1.1 Cyclic lipopeptide deacylase produced by Actinoplanes utahensis
[0028] Using the Actinomycetes uthaensis IFO-13244 strain, according to the fermentation method described in Example 1 of the patent US5376634, the fermentation culture was carried out to obtain 150 L of mycelium culture solution. Then filter through a Buchner funnel covered with filter paper, collect 120 L of filtrate containing cyclolipidase deacylase, that is, the free cyclolipopeptide deacylase solution, collect the filtrate, and detect by HPLC, the enzyme activity is 0.864 × 10 5 U.
[0029] 1.2 Cyclic lipopeptide deacylase produced by Streptomyces sp.
[0030] Streptomyces strain No. 6907 was used to carry out fermentation culture according to the fermentation method described in Example 1 of patent WO97 / 32975 to obtain 150 L of mycelium culture solution. Then filter through a Buchner funnel covered with filter paper...
Embodiment 2
[0033] The selection of embodiment 2 different types of epoxide carriers
[0034] Take the free cyclolipopeptide deacylase solution prepared in 1.1 of Example 1 above, add 1g Eupergit CM or LX1000-EP respectively, stir at 25°C for 24 hours, collect the immobilized cyclolipopeptide deacylase by filtration, and wash with pure water three times , dried at room temperature for 3 hours, and finally carried out the enzyme activity assay.
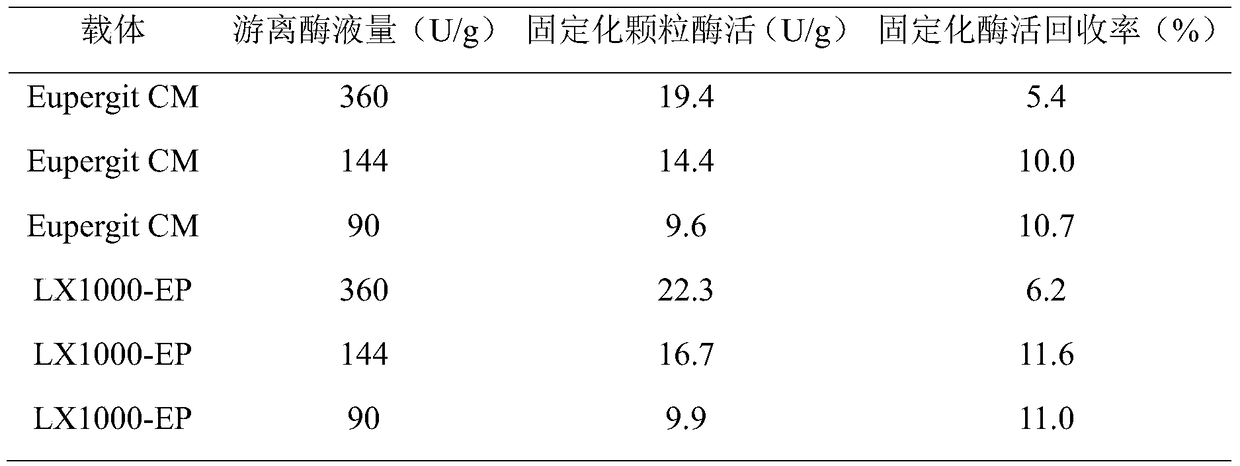
[0035] Table 1 The immobilization of different types of epoxide carriers to the original free enzyme solution
[0036]
[0037] It can be seen from Table 1 that the recovery rate of the vector LX1000-EP is higher than that of the vector EupergitCM under the condition of the same enzyme dosage.
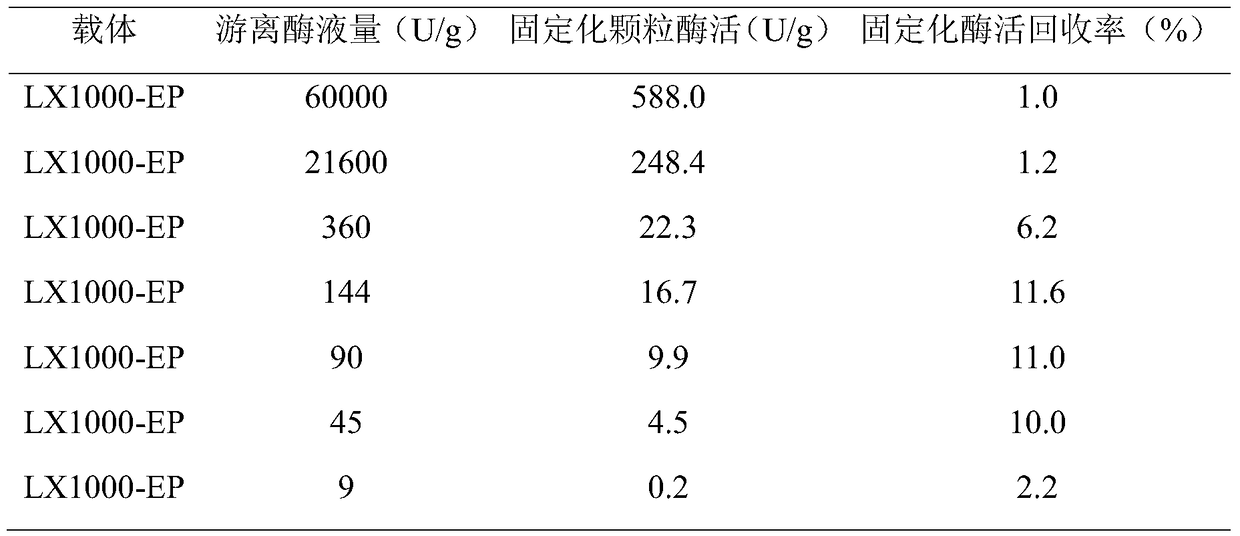
[0038] Table 2 The immobilization of the original free enzyme solution by the epoxide carrier LX1000-EP
[0039]
[0040] It can be seen from Table 2 that the immobilization of the free enzyme can be realized when the mixing ratio of the free cyclo...
Embodiment 3
[0042] The optimization of aqueous solution polarity in the immobilization process of embodiment 3
[0043]Take 1 L of the free cycloaliphatic peptide deacylase solution prepared in 1.1 of Example 1, and add different types of organic solvents (including ethanol, isopropanol, n-hexane, DMSO, acetone, DMF, tetrahydrofuran, pyridine, acetonitrile) respectively. 50mL, 100mL, 200mL or 300mL. Continue to add 5 g of LX1000-EP carrier respectively, stir at 25°C for 24 hours, collect the immobilized cyclolipopeptide deacylase by filtration, wash with pure water three times, dry at room temperature for 3 hours, and finally measure the enzyme activity.
[0044] Table 3 Cyclic lipopeptide deacylase immobilization in different organic solvents
[0045]
[0046]
[0047] As can be seen from Table 3, in the free cycloaliphatic peptide deacylase solution, adding different types of organic solvents, including ethanol, isopropanol, n-hexane, DMSO, acetone, DMF, tetrahydrofuran, pyridine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com