Preparation method and purpose of sodium fluoride sustained-release tablets
A technology of sodium fluoride and sustained-release tablets, which is applied in the field of medical chemistry, can solve the problems of dental fluorosis, poisoning, and the inability of fluoride ions to achieve a therapeutic effect, and achieves the inhibition of dental caries, the sustained-release effect is obvious, and the preparation method is simple and easy to operate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
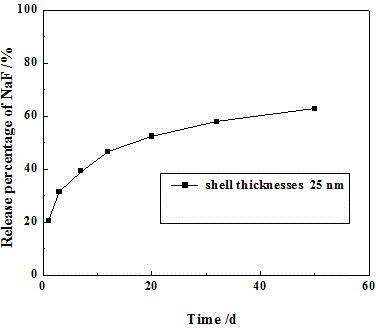
[0032] (1) Preparation of hollow titanium dioxide loaded with sodium fluoride
[0033] To prepare hollow titanium dioxide with a wall thickness of 25nm, weigh 2g of microspheres in a 50mL round bottom flask, slowly add 7.9mL of 0.6mol / L sodium fluoride solution dropwise after vacuuming for 30min, continue vacuuming for 30min, stir, and dry to obtain a loading capacity of 10% Nano Hollow Titanium Dioxide.
[0034] (2) Preparation of Sodium Fluoride Sustained Release Tablets
[0035] Weigh 1g of the prepared hollow titanium dioxide loaded with sodium fluoride into 5mL of Gruma general-purpose binder, stir evenly, pour the mixed solution into the template, and irradiate with a light curing machine to obtain sustained-release tablets with a diameter of 15mm. A disc with a thickness of 2.3mm and a mass of 0.8g.
[0036] (3) In vitro release test of sodium fluoride sustained release agent
[0037] Take two prepared sustained-release tablets and place them in 30mL of artificial sa...
Embodiment 2
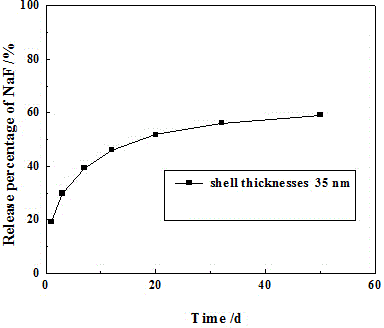
[0039] Hollow titanium dioxide with a wall thickness of 35 nm was prepared, and the method in Example 1 was used to prepare hollow titanium dioxide loaded with sodium fluoride. Weigh 1g of the prepared hollow titanium dioxide loaded with sodium fluoride in 5mL of Gruma general-purpose binder, stir evenly, pour it into the template, and irradiate it with a light curing machine to obtain a slow-release tablet, which is made into a diameter of 15mm and a thickness of 2.3mm, a disc with a mass of 0.8g. Take two prepared slow-release tablets and put them in 30mL of artificial saliva, take 2.0mL samples respectively on the 1d, 3d, 7d, 12d, 20d, 32d, and 50d to measure the fluoride ion concentration, and replace the model tablet after measurement. 30mL of artificial saliva. Add up the released amounts of fluoride ions in the taken solutions to obtain the cumulative F - release amount, recorded as mg / L; from figure 2 It can be seen that from the 1st day to the 50th day, the releas...
Embodiment 3
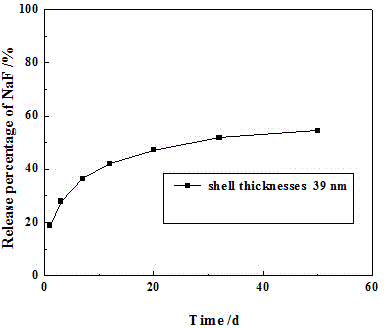
[0041] Hollow titanium dioxide with a wall thickness of 39 nm was prepared, and the method of Example 1 was used to prepare hollow titanium dioxide loaded with sodium fluoride. Weigh 1g of the prepared hollow titanium dioxide loaded with sodium fluoride in 5mL of Gruma general-purpose binder, stir evenly, pour it into the template, and irradiate it with a light curing machine to obtain a slow-release tablet, which is made into a diameter of 15mm and a thickness of 2.3mm, a disc with a mass of 0.8g. Take two prepared slow-release tablets and put them in 30mL of artificial saliva, take 2.0mL samples respectively on the 1d, 3d, 7d, 12d, 20d, 32d, and 50d to measure the fluoride ion concentration, and replace the model tablet after measurement. 30mL of artificial saliva. Add up the released amounts of fluoride ions in the taken solutions to obtain the cumulative F - release amount, recorded as mg / L; from image 3 It can be seen that from the 1st day to the 50th day, the release...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wall thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com