Biodegradable stent composite
A composition and alloy technology, which is applied in medical science, surgery, coating, etc., can solve the problems of no reports and related products that have not been listed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0068] The magnesium alloy composition table that embodiment 1-9 selects is for example as follows:
[0069]
[0070] 8
[0071] Magnesium metal stent materials can also choose high-purity magnesium or high-purity iron according to different degradation times.
Embodiment 1
[0072] Example 1: Vascular covered stent
[0073] Its preparation process is as follows:
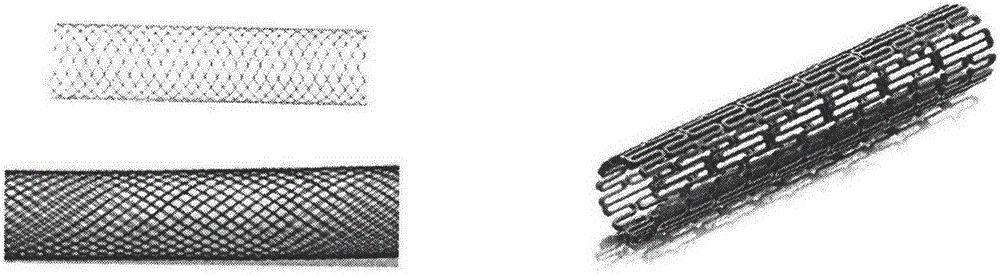
[0074] (1) Prepare, engrave, etch or cut degradable metal materials (alloy composition ratio according to list 9) into required patterns or laths (such as figure 1 Various shapes shown), the diameter of the bracket is 1mm, and the length is 2cm;
[0075] (2) Polish the stent prepared in (1), soak it in 30% hydrofluoric acid for 24 hours, take it out, wash it with 75% ethanol, and dry it.
[0076] (3) Polymer A: degradable medical polyurethane material (viscosity average molecular weight 10,000, breaking strength 35MPa, breaking elongation 350%) is dissolved in chloroform solvent, spreads 0.2-0.3 on the tetrafluoroethylene plate mm thick film;
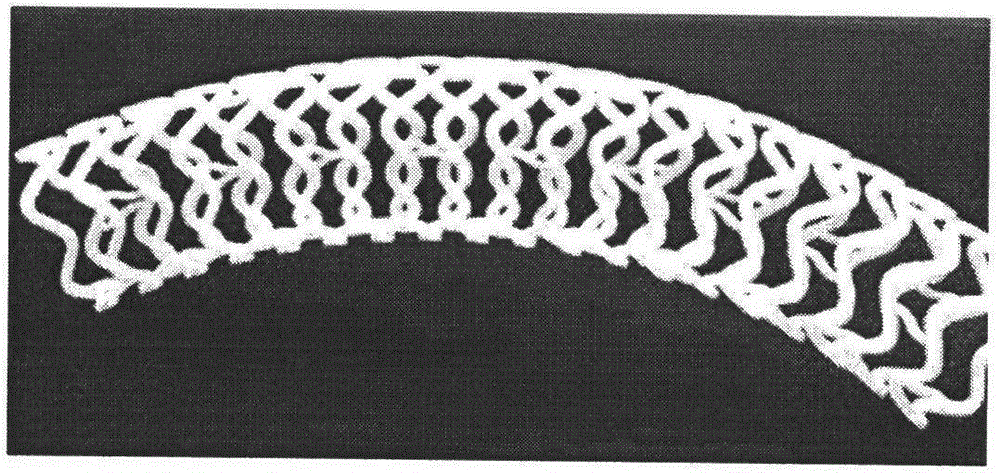
[0077] (4) roll the film prepared in (3) on the metal material surface of the metal stent prepared in (1) to make a covered stent (such as Figure 5 shown);
[0078] (5) The composite material prepared in (4) is dried, polished, and polished ...
Embodiment 2
[0079] Example 2: Double balloon urethral stent graft
[0080] Made of silicone material such as figure 1 In the double-balloon urethral stent shown, the stent is pressed or adhered to the columnar balloon, and after being placed in the urethra, gas is injected into the balloon, and the columnar balloon expands the stent and supports it in the urethra.
[0081] Wherein the peritoneal urethral stent, its preparation process is as follows:
[0082] (1) Prepare, engrave, etch or cut degradable metal materials (alloy composition ratio according to list 9) into required patterns or laths (such as Figure 6 Various shapes shown), the diameter of the bracket is 3mm, and the length is 2cm;
[0083] (2) Polymer A: degradable medical polyurethane material and polymer B: PLGA (wherein LA: GA ratio is 70: 30, viscosity average molecular weight is 80,000) are mixed and dissolved in dichloromethane solvent by 1: 2, Lay a 0.2-0.3mm thick film on the tetrafluoroethylene board;
[0084] (3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com